
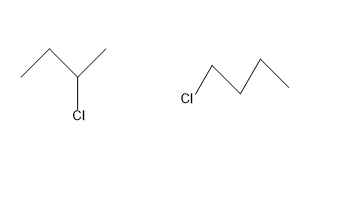
Identify the chiral molecule among the following pair?

Answer
523.2k+ views
Hint: A chiral molecule is the atom which is attached to four different groups or functional groups. It is also known as an asymmetric carbon atom. According to Le-Bel-van't-Hoff rule, the number of stereoisomers of an organic compound can be calculated as\[{2^n}\], where \[n\] is the number of chiral or asymmetric carbon atoms.
Complete answer:
Since, now we have a basic knowledge about chiral atoms. So let’s proceed towards our given option.
Looking at our first option i.e. \[2 - \]chlorobutane – In this molecule as we can see , the carbon atom at which chlorine is attached (marked with red box ) is a asymmetric atom , as it has four different functional group attached to it i.e. \[H,C{H_2},C{H_3},Cl\].
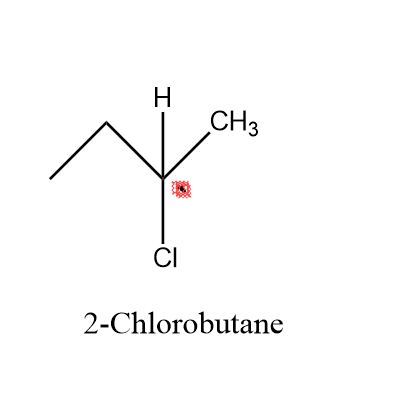
As , we can see clearly in the figure that our second carbon is an asymmetric atom .
Hence, we can say that our \[2 - \]chlorobutane is a chiral molecule.
Coming to our next option \[1 - \] chlorobutane, it is an achiral molecule, as it does not have any asymmetric carbon atom. Each atom is attached to two similar functional groups .
Note:
Phenomenon chirality is a very important concept especially in biology because most of the biological molecules like amino acids, carbohydrates- sugars, starch , cellulose , are chiral in nature and these molecules are the building block for larger molecules like nucleic acid, proteins which is very important for our body composition. All amino acids except glycine are chiral , because it does not contain any chiral centre . Also Chirality is introduced by “Sir William Thomsan” in \[1894\].
Complete answer:
Since, now we have a basic knowledge about chiral atoms. So let’s proceed towards our given option.
Looking at our first option i.e. \[2 - \]chlorobutane – In this molecule as we can see , the carbon atom at which chlorine is attached (marked with red box ) is a asymmetric atom , as it has four different functional group attached to it i.e. \[H,C{H_2},C{H_3},Cl\].

As , we can see clearly in the figure that our second carbon is an asymmetric atom .
Hence, we can say that our \[2 - \]chlorobutane is a chiral molecule.
Coming to our next option \[1 - \] chlorobutane, it is an achiral molecule, as it does not have any asymmetric carbon atom. Each atom is attached to two similar functional groups .
Note:
Phenomenon chirality is a very important concept especially in biology because most of the biological molecules like amino acids, carbohydrates- sugars, starch , cellulose , are chiral in nature and these molecules are the building block for larger molecules like nucleic acid, proteins which is very important for our body composition. All amino acids except glycine are chiral , because it does not contain any chiral centre . Also Chirality is introduced by “Sir William Thomsan” in \[1894\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




