
Identify product when R- and (S)-2-butanol reacts with (R, R) tartaric acid in acidic medium.
(A) Racemic
(B) Diastereomers
(C) Meso
(D) Pure enantiomer
Answer
568.8k+ views
Hint: The R, R-tartaric acid reacts with R and S-butanol . The reaction proceeds with the removal of water molecules. The hydrogen atom of tartaric acid and the hydroxyl group of the butanol is removed as a water molecule to form new stereocenters.
Complete Solution :
The stereoisomers which do not form mirror images of each other and non –superimposable on one another with its stereocenters are called the diastereomers.
The (R, R) tartaric acid and the R and S- 2-butanol enantiomers react with each other.
1) When R-2-Butanol reacts with (R, R) –tartaric acid we get a product as shown below:
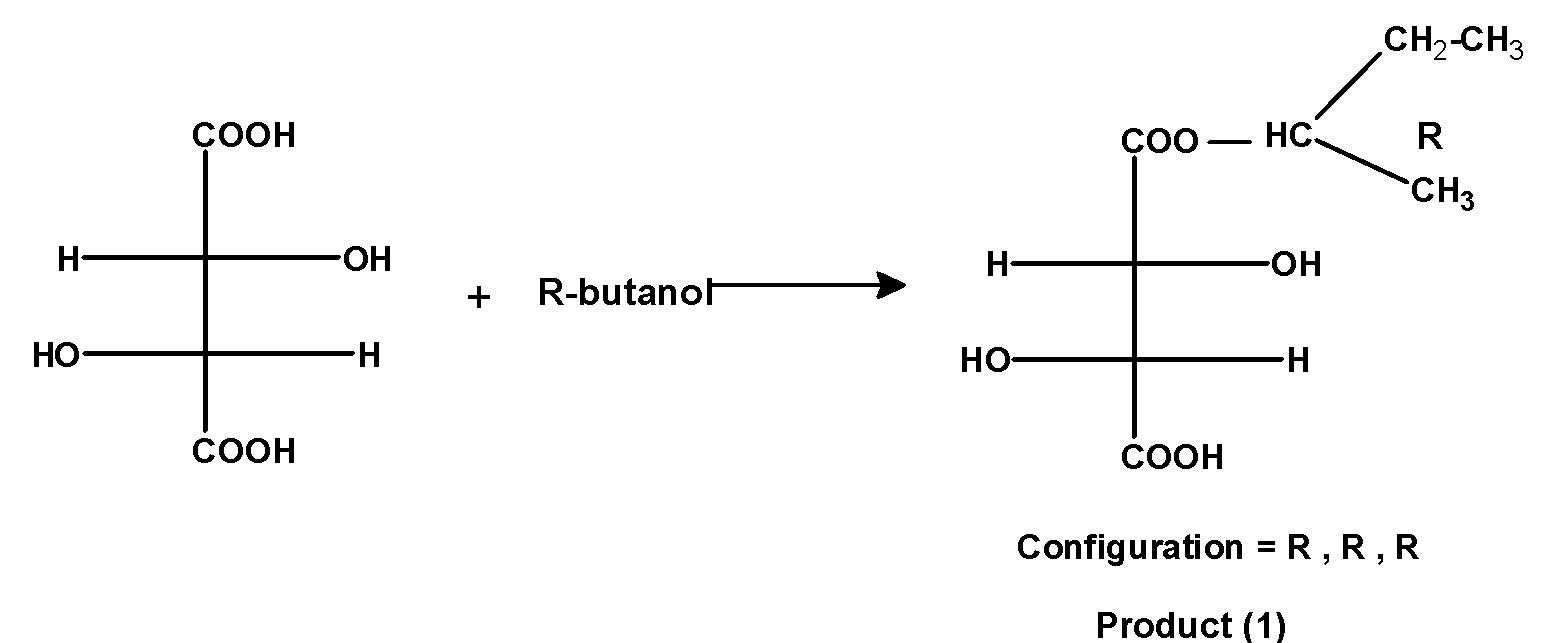
The hydrogen atom of the tartaric acid and the hydroxyl group of R-butanol is removed as a water molecule. The resultant product has three stereocenters. All stereocenters have an R configuration.
2) When S-2-Butanol reacts with (R, R) –tartaric acid we get a product as shown below:
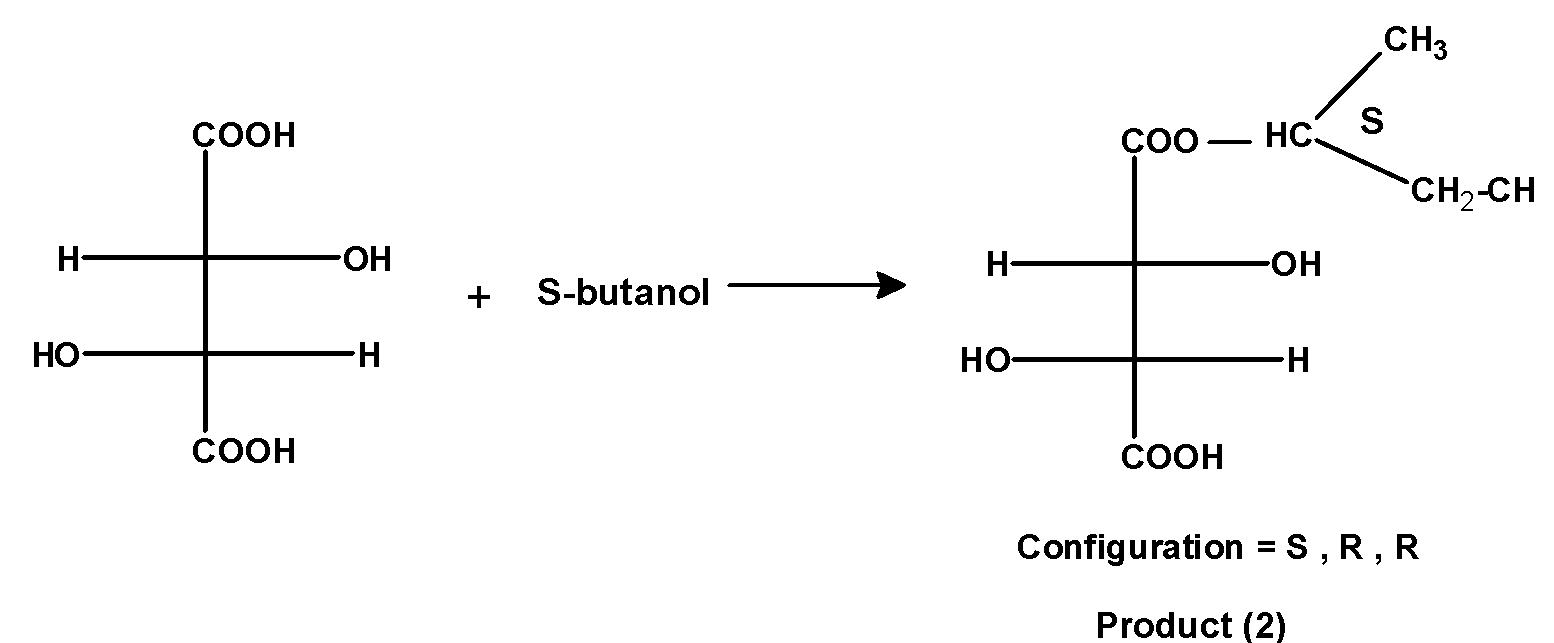
The hydrogen atom of the tartaric acid and the hydroxyl group of R-butanol is removed as a water molecule. The resultant product has three stereocenters. Here two stereocenters (From the tartaric acid) have an R configuration while the other stereocenters formed by the removal of water have an S configuration.
The product (1) and (2) have three stereocenters .Out of those two stereocenters are the same and they differ at only one stereocenter.The mirror image of the product (1) is not a mirror image of the product (2).
Since products (1) and (2) are not mirror images of each other. Therefore, products (1) and (2) are diastereoisomers.
So, the correct answer is “Option B”.
Note: Note that, diastereomers can be easily determined by looking at the designation or the configuration. The molecule which has the same or a designation is non-identical are diastereomers. For example, (R, R, R) and (R, S, R), etc.
Complete Solution :
The stereoisomers which do not form mirror images of each other and non –superimposable on one another with its stereocenters are called the diastereomers.
The (R, R) tartaric acid and the R and S- 2-butanol enantiomers react with each other.
1) When R-2-Butanol reacts with (R, R) –tartaric acid we get a product as shown below:

The hydrogen atom of the tartaric acid and the hydroxyl group of R-butanol is removed as a water molecule. The resultant product has three stereocenters. All stereocenters have an R configuration.
2) When S-2-Butanol reacts with (R, R) –tartaric acid we get a product as shown below:

The hydrogen atom of the tartaric acid and the hydroxyl group of R-butanol is removed as a water molecule. The resultant product has three stereocenters. Here two stereocenters (From the tartaric acid) have an R configuration while the other stereocenters formed by the removal of water have an S configuration.
The product (1) and (2) have three stereocenters .Out of those two stereocenters are the same and they differ at only one stereocenter.The mirror image of the product (1) is not a mirror image of the product (2).
Since products (1) and (2) are not mirror images of each other. Therefore, products (1) and (2) are diastereoisomers.
So, the correct answer is “Option B”.
Note: Note that, diastereomers can be easily determined by looking at the designation or the configuration. The molecule which has the same or a designation is non-identical are diastereomers. For example, (R, R, R) and (R, S, R), etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




