
identify ‘A’ in the following reaction:
\[{\text{A + 2 Na }}\xrightarrow[{{\text{ether}}}]{{{\text{Dry}}}}{\text{ 2,2,5,5 - tetramethylhexane}}\] + 2 NaBr
A ) 2 - bromo - 2 - methylbutane
B ) 1 - bromo - 2,2 - dimethylpropane
C ) 1 - bromo - 3 - methylbutane
D ) 1 - Bromo - 2methylpropane
Answer
585.6k+ views
Hint: In presence of sodium metal, two molecules of alkyl halide condense to form a bigger hydrocarbon. Two molecules of sodium halide are the byproduct. The number of carbon atoms in the product is twice the number of carbon atoms in the starting material.
Complete step by step answer:
Write the structure of 2,2,5,5 - tetramethylhexane as shown below:
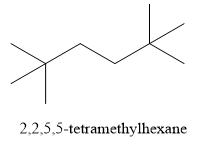
Let us find the solution by writing reactions:
A) Write the reaction of 2-bromo-2-methylbutane:
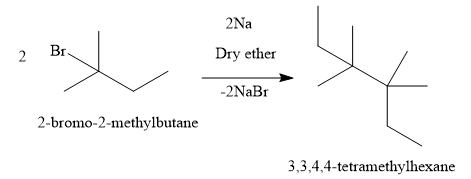
The compound formed is 3,3,4,4-tetramethylhexane which is not the correct answer.
B) Write the reaction of 1 - bromo - 2,2 - dimethylpropane with sodium to form 2,2,5,5 - tetramethylhexane as shown below:
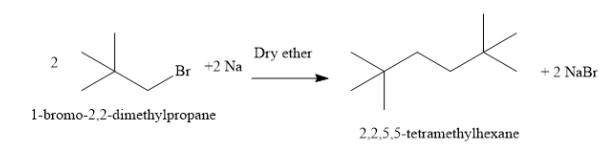
Two molecules of 1 - bromo - 2,2 - dimethylpropane condense to form one molecule of 2,2,5,5 - tetramethylhexane. A new carbon-carbon bond is formed in the reaction. The reaction is carried out in the solvent dry ether. The reagent is sodium metal. Sodium metal combines with bromine atoms and forms sodium bromide. In this reaction, an alkyl halide is converted to higher alkane.
Thus, the compound A is 1 - bromo - 2,2 - dimethylpropane.
Hence, the option B ) is the correct option.
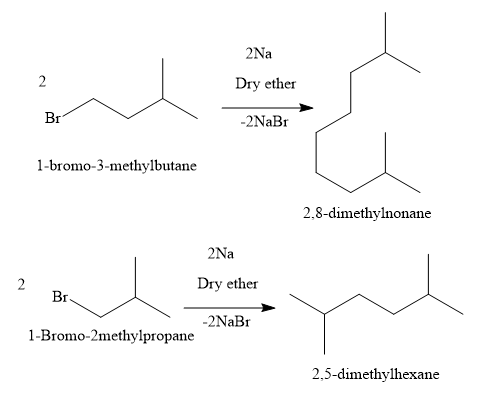
Additional Information: Write the reactions of the substrates of other options:
Note: If the reaction is carried out using a mixture of two alkyl halides, then unsymmetrical higher .alkane can be obtained as the product through cross coupling by coupling of two molecules of different alkyl halides. However, it will also contain the other byproducts which are symmetrical higher alkanes, obtained by coupling of two molecules of the same alkyl halide. Hence, this method is suitable for the formation of symmetrical higher alkanes. But this method is not suitable for the formation of unsymmetrical higher alkanes.
Complete step by step answer:
Write the structure of 2,2,5,5 - tetramethylhexane as shown below:

Let us find the solution by writing reactions:
A) Write the reaction of 2-bromo-2-methylbutane:

The compound formed is 3,3,4,4-tetramethylhexane which is not the correct answer.
B) Write the reaction of 1 - bromo - 2,2 - dimethylpropane with sodium to form 2,2,5,5 - tetramethylhexane as shown below:

Two molecules of 1 - bromo - 2,2 - dimethylpropane condense to form one molecule of 2,2,5,5 - tetramethylhexane. A new carbon-carbon bond is formed in the reaction. The reaction is carried out in the solvent dry ether. The reagent is sodium metal. Sodium metal combines with bromine atoms and forms sodium bromide. In this reaction, an alkyl halide is converted to higher alkane.
Thus, the compound A is 1 - bromo - 2,2 - dimethylpropane.
Hence, the option B ) is the correct option.
Additional Information: Write the reactions of the substrates of other options:

Note: If the reaction is carried out using a mixture of two alkyl halides, then unsymmetrical higher .alkane can be obtained as the product through cross coupling by coupling of two molecules of different alkyl halides. However, it will also contain the other byproducts which are symmetrical higher alkanes, obtained by coupling of two molecules of the same alkyl halide. Hence, this method is suitable for the formation of symmetrical higher alkanes. But this method is not suitable for the formation of unsymmetrical higher alkanes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




