
Identify A and B in the following reactions
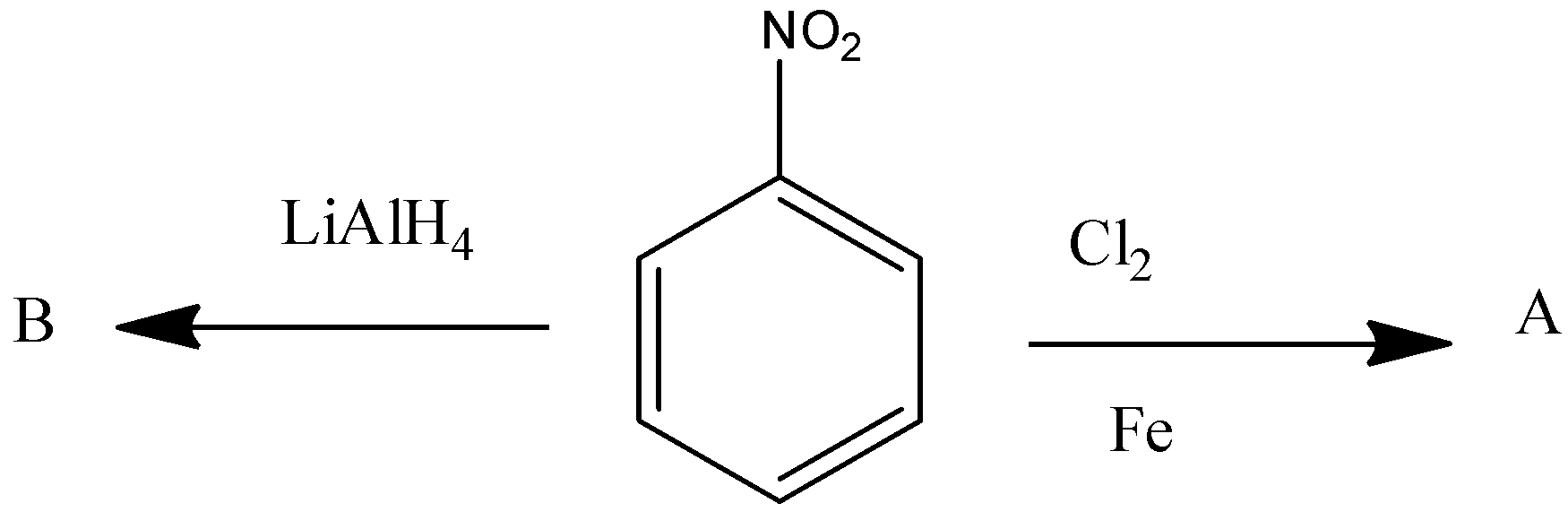

Answer
566.7k+ views
Hint: Benzene is an aromatic non-polar compound that gets attached to by various groups altering its properties and forming new compounds. The various groups being attached to the benzene structure can be electron-withdrawing or electron-donating which guides the properties of the compound formed and its reactions with various elements and reagents.
Complete step by step answer:
Benzene is an organic planar compound that has the formula of ${C_6}{H_6}$ and has 6 carbons attached in the ring in a plane. The electrons in the compounds are delocalized and thus show aromatic properties.
The secondary compound that attaches to benzene may be at one of the three positions of ortho, meta, and para. Which are the relative positions concerning another main substituent already attached to the benzene.
The compound that is attached to benzene in the above question is the nitro $( - N{O_2})$ compound and is a strong electron-withdrawing in nature. It is also found that the nitro group is meta directing means it directs any secondary group to meta position concerning its position.
Considering the first reaction,
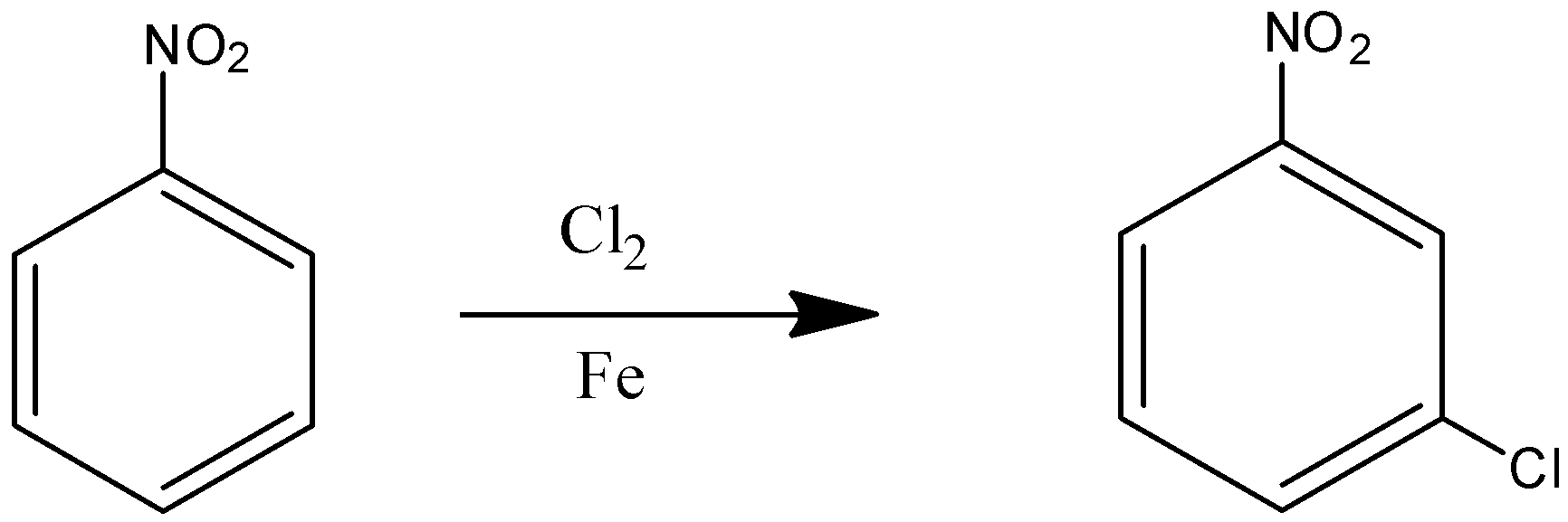
Here nitrobenzene is chlorinated thus forming meta chloro nitrobenzene, which has chlorine attached to the meta position concerning the nitro group.
Considering the second reaction,
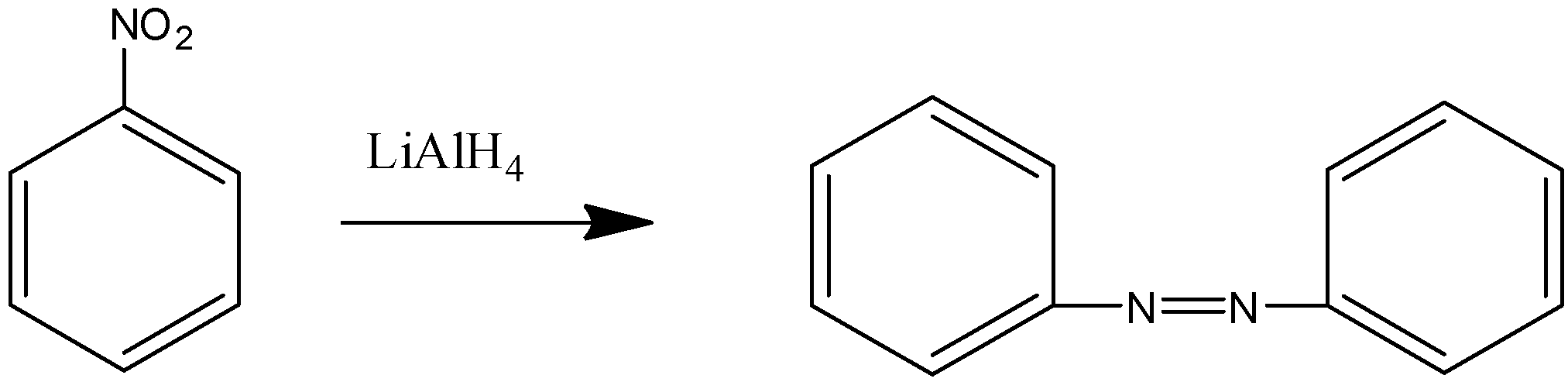
Nitro benzene on reaction with lithium aluminum hydride gives azobenzene.
$LiAl{H_4}$ is a well-known reducing agent and is responsible for the reduction of the nitro group on the benzene thus forming azodyes.
So, A is meta chlorobenzene and B is azo dye.
Note: Lithium aluminum hydride abbreviated as LAH is a grey solid which is used as a reducing agent in various reactions.It is highly reactive towards water releasing hydrogen gas.Azo Dyes are usually used in the textile industry for treating leather, textiles, and some foods.
Complete step by step answer:
Benzene is an organic planar compound that has the formula of ${C_6}{H_6}$ and has 6 carbons attached in the ring in a plane. The electrons in the compounds are delocalized and thus show aromatic properties.
The secondary compound that attaches to benzene may be at one of the three positions of ortho, meta, and para. Which are the relative positions concerning another main substituent already attached to the benzene.
The compound that is attached to benzene in the above question is the nitro $( - N{O_2})$ compound and is a strong electron-withdrawing in nature. It is also found that the nitro group is meta directing means it directs any secondary group to meta position concerning its position.
Considering the first reaction,

Here nitrobenzene is chlorinated thus forming meta chloro nitrobenzene, which has chlorine attached to the meta position concerning the nitro group.
Considering the second reaction,

Nitro benzene on reaction with lithium aluminum hydride gives azobenzene.
$LiAl{H_4}$ is a well-known reducing agent and is responsible for the reduction of the nitro group on the benzene thus forming azodyes.
So, A is meta chlorobenzene and B is azo dye.
Note: Lithium aluminum hydride abbreviated as LAH is a grey solid which is used as a reducing agent in various reactions.It is highly reactive towards water releasing hydrogen gas.Azo Dyes are usually used in the textile industry for treating leather, textiles, and some foods.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE

Calculate the equivalent resistance between a and b class 12 physics CBSE

How many states of matter are there in total class 12 chemistry CBSE

Which of the following is the best conductor of electricity class 12 physics CBSE




