
$(i)$\[C{O_2}\] is linear where $S{O_2}$ is bend shaped.
$(ii)$${H_2}^ + $ ions are more stable than ${H_2}^ - $ though they have same bond order
Answer
501k+ views
Hint: The geometry of the compound depends on the different numbers of electrons in each molecule and the VSEPR (Valence shell electron repulsion) theory. This theory states that the electrons are negatively charged and the valence electrons in different atoms in a molecule repel each other.
Complete answer:
$(i)$ According to the valence shell electron pair repulsion (VSPER) theory, if all the electron pairs around the central atom are not bonded then, the lone pairs distort the shape of the molecule due to the repulsion. In \[C{O_2}\], carbon has $4$ electrons in its outermost shell, and oxygen has $6$ electrons in its valence shell. The carbon is in the center because of its lower electronegativity, there are no lone pairs present on the atom of carbon, so there will be less repulsion. Therefore, \[C{O_2}\] is a linear structure.
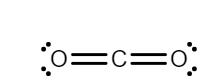
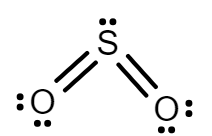
While in $S{O_2}$, Sulphur has $6$ electrons in its valence shell and after forming a bond with oxygen, Sulphur leftover with $1$ lone pair. Hence there will be repulsion between bond pairs and lone pairs. So, due to the repulsion structure of $S{O_2}$ is bent.
$(ii)$ The configuration of ${H_2}^ + $ is one electron in $1s$ bonding orbital, whereas the electronic configuration of ${H_2}^ - $ is two electrons in $1s$ bonding orbital and $1$ electron in $1s$ antibonding orbital. So you can say that the bond order for both is $\dfrac{1}{2}$. But since ${H_2}^ - $ has more antibonding electrons it will be less stable than ${H_2}^ + $ because electrons in antibonding orbitals decrease the stability of the molecule. So ${H_2}^ + $ is more stable than ${H_2}^ - $.
Note:
Lone pair takes up more space than bonding electrons, as they are only attached to one atom rather than two, so they repel more than bonding electrons. Therefore we can order repulsions between different types of electron pairs: Lone pair-lone pair $ > $ bonding pair-lone pair $ > $ bonding pair-bonding pair.
The formula for calculating the bond order is:
Bond order= $\dfrac{{\left( {No.{\text{ }}of{\text{ }}electrons{\text{ }}in{\text{ }}antibonding{\text{ }}Molecular{\text{ }}orbit} \right) - \;\left( {No.{\text{ }}of{\text{ }}electrons{\text{ }}in{\text{ }}bonding{\text{ }}molecular{\text{ }}orbit} \right)}}{2}$
Complete answer:
$(i)$ According to the valence shell electron pair repulsion (VSPER) theory, if all the electron pairs around the central atom are not bonded then, the lone pairs distort the shape of the molecule due to the repulsion. In \[C{O_2}\], carbon has $4$ electrons in its outermost shell, and oxygen has $6$ electrons in its valence shell. The carbon is in the center because of its lower electronegativity, there are no lone pairs present on the atom of carbon, so there will be less repulsion. Therefore, \[C{O_2}\] is a linear structure.
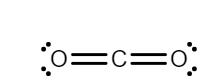
While in $S{O_2}$, Sulphur has $6$ electrons in its valence shell and after forming a bond with oxygen, Sulphur leftover with $1$ lone pair. Hence there will be repulsion between bond pairs and lone pairs. So, due to the repulsion structure of $S{O_2}$ is bent.

$(ii)$ The configuration of ${H_2}^ + $ is one electron in $1s$ bonding orbital, whereas the electronic configuration of ${H_2}^ - $ is two electrons in $1s$ bonding orbital and $1$ electron in $1s$ antibonding orbital. So you can say that the bond order for both is $\dfrac{1}{2}$. But since ${H_2}^ - $ has more antibonding electrons it will be less stable than ${H_2}^ + $ because electrons in antibonding orbitals decrease the stability of the molecule. So ${H_2}^ + $ is more stable than ${H_2}^ - $.
Note:
Lone pair takes up more space than bonding electrons, as they are only attached to one atom rather than two, so they repel more than bonding electrons. Therefore we can order repulsions between different types of electron pairs: Lone pair-lone pair $ > $ bonding pair-lone pair $ > $ bonding pair-bonding pair.
The formula for calculating the bond order is:
Bond order= $\dfrac{{\left( {No.{\text{ }}of{\text{ }}electrons{\text{ }}in{\text{ }}antibonding{\text{ }}Molecular{\text{ }}orbit} \right) - \;\left( {No.{\text{ }}of{\text{ }}electrons{\text{ }}in{\text{ }}bonding{\text{ }}molecular{\text{ }}orbit} \right)}}{2}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




