
(i) Ethanoic acid to ethanamine
(ii) p-toluidine to 2-bromo-4-methylaniline.
(iii) methyl bromide to ethanamine
Answer
566.7k+ views
Hint: We can make any compound to undergo conversion into any corresponding alcohol, acid ,ether or amine only then, if we know about those compounds properly, their methods of preparation, their physical-chemical properties and their chemical reactions. So, recall the reactions of the above given compounds properly and then , you can easily make their conversion into the corresponding compounds.
Complete answer:
Considering the given conversions one by one as:
(i) Ethanoic acid to ethanamine
First of all, ethanoic acid undergoes reduction to alcohols in the [presence of lithium aluminum hydride and then alcohols so formed when made to react with the metal halides i.e. phosphorus pentachloride, forms alkyl halide when on reaction with ammonia gives the ethanamine. The overall reaction occurs as;
$C{{H}_{3}}COOH\xrightarrow{LiAl{{H}_{4}}}C{{H}_{3}}C{{H}_{2}}OH\xrightarrow{PC{{l}_{5}}}C{{H}_{3}}C{{H}_{2}}Cl\xrightarrow{N{{H}_{3}}}C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$
(ii) p-toluidine to 2-bromo-4-methylaniline.
In this, first of all p-toluidine reacts with the acid chloride and bromine and results in the formation of the compound which on hydrolysis results in the formation of the 2-bromo-4-methylaniline. The reaction occurs as:
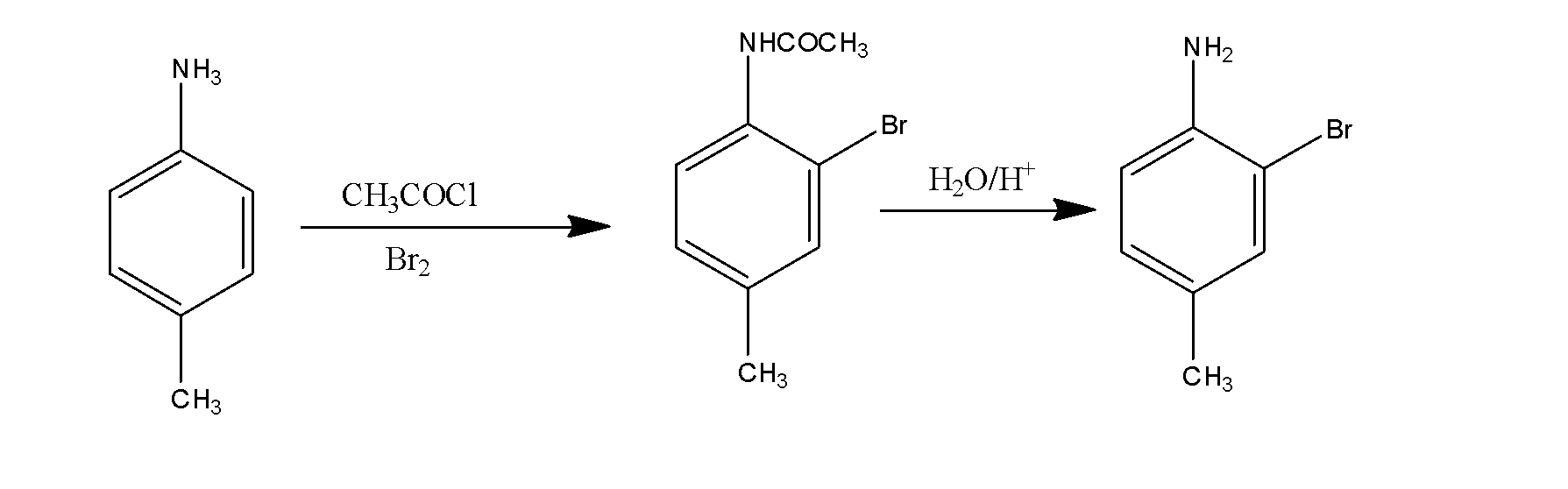
(iii) methyl bromide to ethanamine
In this, first of all the methyl bromide reacts with potassium cyanide and results in the formation of the alkyl cyanide i.e. methyl cyanide and when this is made to react with the ethyl alcohol in the presence of sodium, it forms the amine i.e. the ethanamine. The overall reaction occurs as;
$C{{H}_{3}}Br\xrightarrow{KCN}C{{H}_{3}}CN\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$
Note: The reaction of the alkyl cyanide with the reagent alcohol i.e. the ethanol in the presence of metal i.e. Na results in the reduction of alkyl cyanide into the primary amines and this reaction is known as the Medius reaction.
Complete answer:
Considering the given conversions one by one as:
(i) Ethanoic acid to ethanamine
First of all, ethanoic acid undergoes reduction to alcohols in the [presence of lithium aluminum hydride and then alcohols so formed when made to react with the metal halides i.e. phosphorus pentachloride, forms alkyl halide when on reaction with ammonia gives the ethanamine. The overall reaction occurs as;
$C{{H}_{3}}COOH\xrightarrow{LiAl{{H}_{4}}}C{{H}_{3}}C{{H}_{2}}OH\xrightarrow{PC{{l}_{5}}}C{{H}_{3}}C{{H}_{2}}Cl\xrightarrow{N{{H}_{3}}}C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$
(ii) p-toluidine to 2-bromo-4-methylaniline.
In this, first of all p-toluidine reacts with the acid chloride and bromine and results in the formation of the compound which on hydrolysis results in the formation of the 2-bromo-4-methylaniline. The reaction occurs as:

(iii) methyl bromide to ethanamine
In this, first of all the methyl bromide reacts with potassium cyanide and results in the formation of the alkyl cyanide i.e. methyl cyanide and when this is made to react with the ethyl alcohol in the presence of sodium, it forms the amine i.e. the ethanamine. The overall reaction occurs as;
$C{{H}_{3}}Br\xrightarrow{KCN}C{{H}_{3}}CN\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$
Note: The reaction of the alkyl cyanide with the reagent alcohol i.e. the ethanol in the presence of metal i.e. Na results in the reduction of alkyl cyanide into the primary amines and this reaction is known as the Medius reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




