
Hyper conjugation is possible in which of the following species?
A. ${\text{C}}{{\text{H}}_{\text{3}}} - {\text{CH}}_{\text{2}}^{\text{ + }}$
B. ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\, - \,{\text{C}}{{\text{H}}_{\text{3}}}$
C. ${\text{C}}{{\text{H}}_2} = {\text{C}}{{\text{H}}_2}$
D. ${\text{C}}{{\text{H}}_3}\, - {\text{C}} - {({\text{C}}{{\text{H}}_3})_2} - {\text{CH}} = {\text{C}}{{\text{H}}_2}$
Answer
559.8k+ views
Hint: To determine the answer we should know what hyperconjugation is. In Hyper conjugation hydrogen from an alkyl transfer to the unsaturated carbon. We will find the species in which alkyl is present nearby unsaturation.
Complete step-by-step answer:
During Hyper conjugation hydrogen of sigma bond or alkyl hydrogen transfer to the carbon having unsaturation means double or triple bond. For Hyper conjugation, two carbon having ${\text{s}}{{\text{p}}^{\text{3}}}$and ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization should be adjacent.
The interaction between orbitals during hyper conjugation is shown as follows:
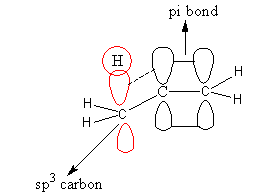
So, we determine the species having ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized carbon atom near to the double bond.
The given structures are as follows:
${\text{C}}{{\text{H}}_{\text{3}}} - {\text{CH}}_{\text{2}}^{\text{ + }}$
In the above structure unsaturation is not present so, it will not show hyper-conjugation.
${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\, - \,{\text{C}}{{\text{H}}_{\text{3}}}$
In the above structure phenyl ring have unsaturation and hence the ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised carbon centres and it also have methyl group whose hybridization is ${\text{s}}{{\text{p}}^{\text{3}}}$so, it will show hyper-conjugation.
The hyper-conjugation is shown as follows:
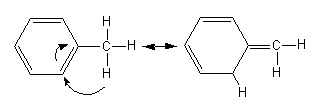
${\text{C}}{{\text{H}}_2} = {\text{C}}{{\text{H}}_2}$
In the above species unsaturation is present but alkyl group or ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon centre with hydrogen is not present so, it will not show hyper conjugation.
D. ${\text{C}}{{\text{H}}_3}\, - {\text{C}} - {({\text{C}}{{\text{H}}_3})_2} - {\text{CH}} = {\text{C}}{{\text{H}}_2}$ In the above species unsaturation is present and alkyl group or ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon centre is also present it does not has any hydrogen that can transfer present so, it will not show hyper conjugation.
So, hyper conjugation is possible in ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\, - \,{\text{C}}{{\text{H}}_{\text{3}}}$species.
Therefore, option (B) ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\,\,-\,\,{\text{C}}{{\text{H}}_{\text{3}}}$is correct.
Note: The unsaturation and alkyl group are must for hybridization. During hybridization sigma bond form alkyl breaks and sigma bond with unsaturated carbon forms. The alkyl group are ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised. Only the presence of ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon is not sufficient, the ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon should have at least one hydrogen.
Complete step-by-step answer:
During Hyper conjugation hydrogen of sigma bond or alkyl hydrogen transfer to the carbon having unsaturation means double or triple bond. For Hyper conjugation, two carbon having ${\text{s}}{{\text{p}}^{\text{3}}}$and ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization should be adjacent.
The interaction between orbitals during hyper conjugation is shown as follows:

So, we determine the species having ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized carbon atom near to the double bond.
The given structures are as follows:
${\text{C}}{{\text{H}}_{\text{3}}} - {\text{CH}}_{\text{2}}^{\text{ + }}$
In the above structure unsaturation is not present so, it will not show hyper-conjugation.
${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\, - \,{\text{C}}{{\text{H}}_{\text{3}}}$
In the above structure phenyl ring have unsaturation and hence the ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised carbon centres and it also have methyl group whose hybridization is ${\text{s}}{{\text{p}}^{\text{3}}}$so, it will show hyper-conjugation.
The hyper-conjugation is shown as follows:

${\text{C}}{{\text{H}}_2} = {\text{C}}{{\text{H}}_2}$
In the above species unsaturation is present but alkyl group or ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon centre with hydrogen is not present so, it will not show hyper conjugation.
D. ${\text{C}}{{\text{H}}_3}\, - {\text{C}} - {({\text{C}}{{\text{H}}_3})_2} - {\text{CH}} = {\text{C}}{{\text{H}}_2}$ In the above species unsaturation is present and alkyl group or ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon centre is also present it does not has any hydrogen that can transfer present so, it will not show hyper conjugation.
So, hyper conjugation is possible in ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\, - \,{\text{C}}{{\text{H}}_{\text{3}}}$species.
Therefore, option (B) ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\,\,-\,\,{\text{C}}{{\text{H}}_{\text{3}}}$is correct.
Note: The unsaturation and alkyl group are must for hybridization. During hybridization sigma bond form alkyl breaks and sigma bond with unsaturated carbon forms. The alkyl group are ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised. Only the presence of ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon is not sufficient, the ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised carbon should have at least one hydrogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




