
Hydrolysis of trichloromethane with aqueous KOH gives?
(A) acetic acid
(B) methanol
(C) formic acid
(D) ethanol
Answer
572.1k+ views
Hint: Understand the action of aqueous KOH when the reactant is alkyl halide. Chlorine atoms are replaced by hydroxide ions through the ${{S}_{N}}1$ mechanism. It is important to note that two hydroxyl groups cannot exist on 1 carbon atom. Instead it leads to formation of a carbonyl group by releasing 1 molecule of water.
Complete step by step answer:
Aqueous KOH is commonly used in organic chemistry for the addition of hydroxide to an alkyl halide. The halogen atom is replaced by the incoming hydroxide ions
We will now draw the expanded structure of the compound, trichloromethane.
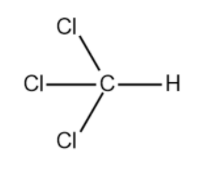
When KOH is added to a solution containing trichloromethane, a substitution reaction takes place. So, the 3 chlorine atoms present in the compound are replaced by 3 hydroxide ions.
However, we know that 2 hydroxide ions cannot exist on the same carbon atom. In order to stabilise the compound, the two hydroxide ions combine to form a carbonyl group and release a molecule of water.
The reaction is given below:
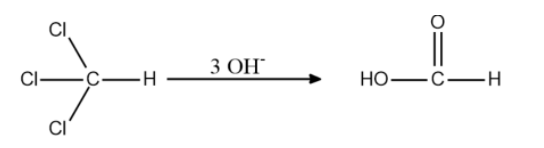
From the above statements and reactions, we can conclude that the hydrolysis of trichloromethane with aqueous KOH gives methanoic acid as the product.
Therefore, the correct answer is option (C).
Note: It is important to know that formic acid and methanoic acid are names for the carboxylic acid compound. The IUPAC name of the compound is methanoic acid. However, the common name for the compound is formic acid.
Complete step by step answer:
Aqueous KOH is commonly used in organic chemistry for the addition of hydroxide to an alkyl halide. The halogen atom is replaced by the incoming hydroxide ions
We will now draw the expanded structure of the compound, trichloromethane.

When KOH is added to a solution containing trichloromethane, a substitution reaction takes place. So, the 3 chlorine atoms present in the compound are replaced by 3 hydroxide ions.
However, we know that 2 hydroxide ions cannot exist on the same carbon atom. In order to stabilise the compound, the two hydroxide ions combine to form a carbonyl group and release a molecule of water.
The reaction is given below:

From the above statements and reactions, we can conclude that the hydrolysis of trichloromethane with aqueous KOH gives methanoic acid as the product.
Therefore, the correct answer is option (C).
Note: It is important to know that formic acid and methanoic acid are names for the carboxylic acid compound. The IUPAC name of the compound is methanoic acid. However, the common name for the compound is formic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




