
Hydrolysis of
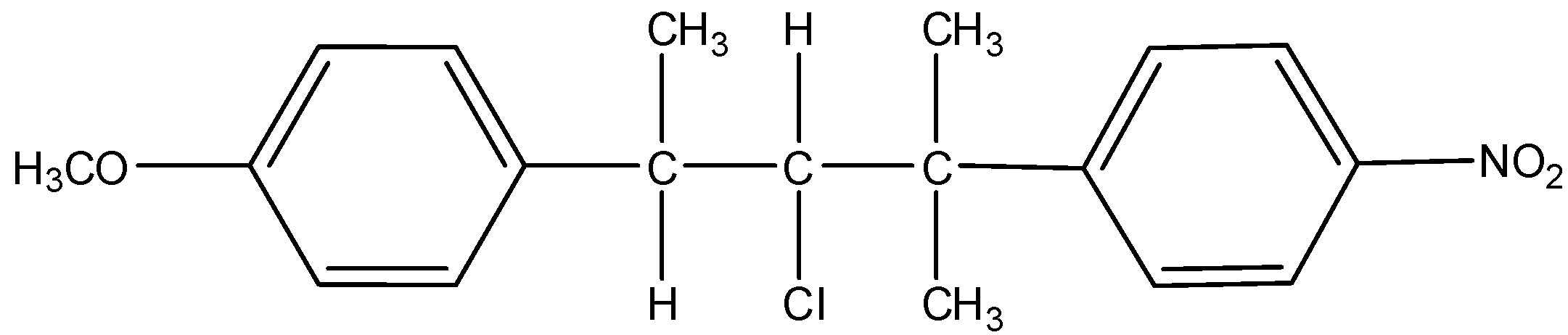
in aqueous acetone medium gives the following products:
K =
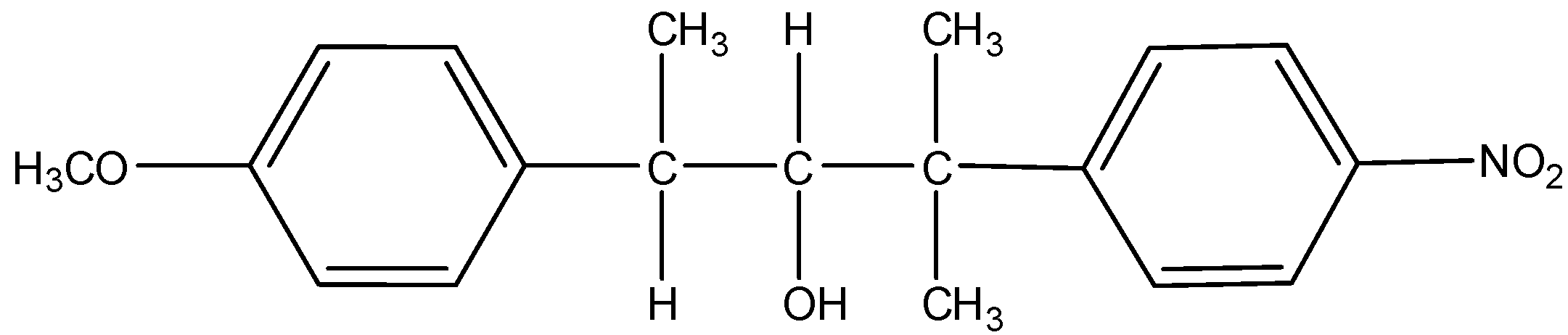
L =
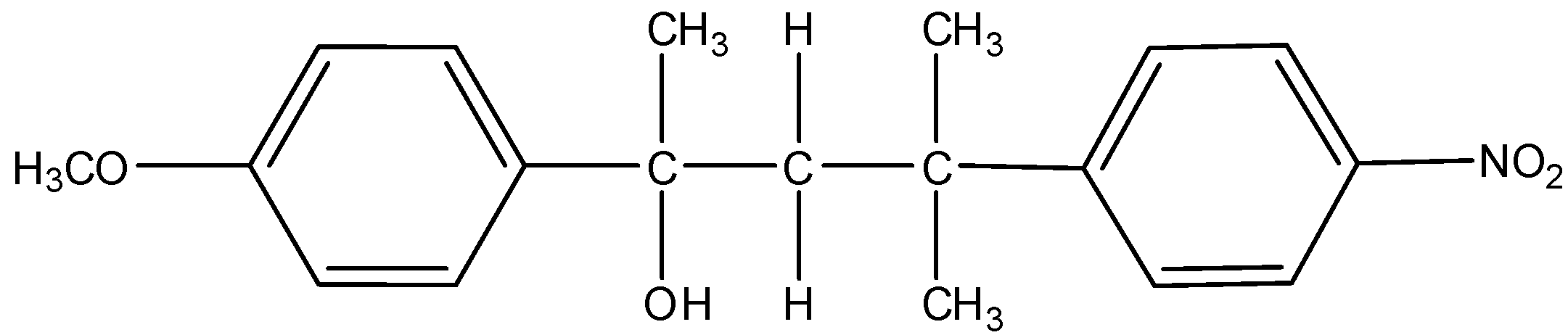
M =
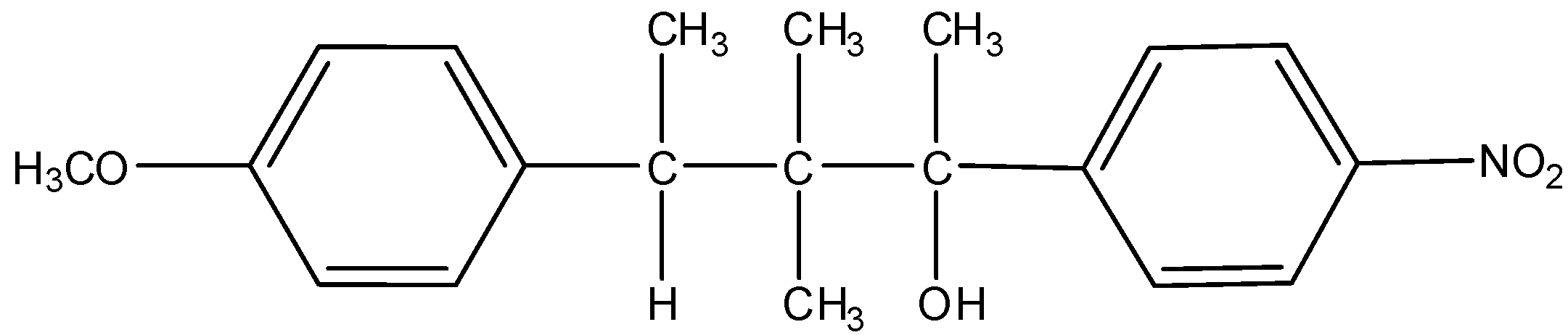
(A) K and L
(B) Only K
(C) L and M
(D) Only M




Answer
579.3k+ views
Hint: A substitution reaction will occur here. In polar solvents, ${S_N}1$ types of reactions are more favoured especially in the compounds which can form stable carbocations. Hydride shift occurs in one of the intermediates in this reaction.
Complete answer:
We are given a compound and we need to find the product of its aqueous hydrolysis. The solvent medium is of aqueous acetone here.
- From the structure of the compound, we can say that ether group and nitro group will be stable and will not react with aqueous acetone. The chlorine atom will be a reactive site in the compound.
- As the reaction medium contains water which is a polar solvent and the chlorine bearing carbon is a secondary carbon, both will favor ${S_N}1$ type of reaction. So, chlorine will leave and form a carbocation. This carbocation will be attacked by the nucleophile $H{O^ - }$ of the water. This will substitute –OH group in place of –Cl. The mechanism can be given as:
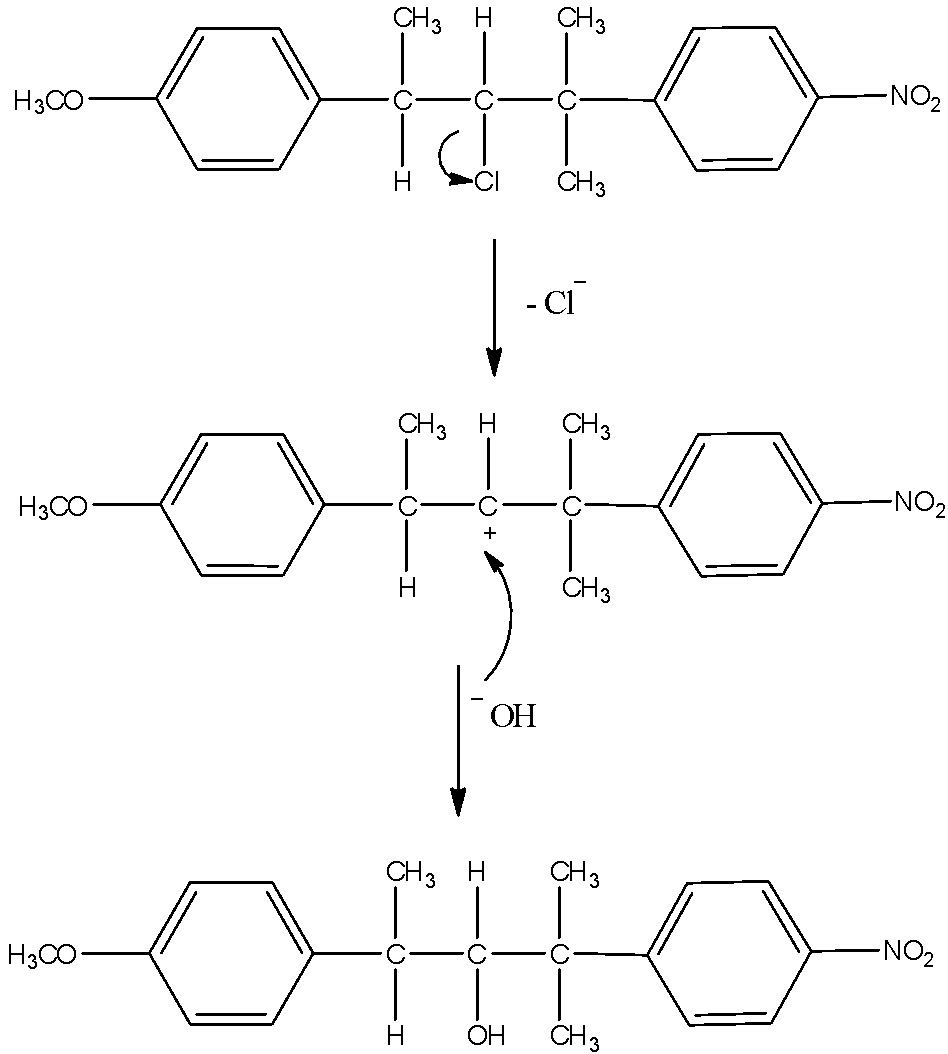
Now, another possibility is also there. The carbocation formed can undergo hydride shift to make different carbocation. That will give rise to another product. Here, the possibility is that either hydrogen atom shifts of methyl group shifts. Hydrogen atoms always favor migration in comparison with methyl groups. So, that and the formation of new more stable benzylic carbocation is the reason why hydride shift occurs here. The mechanism of the formation of another product is here.
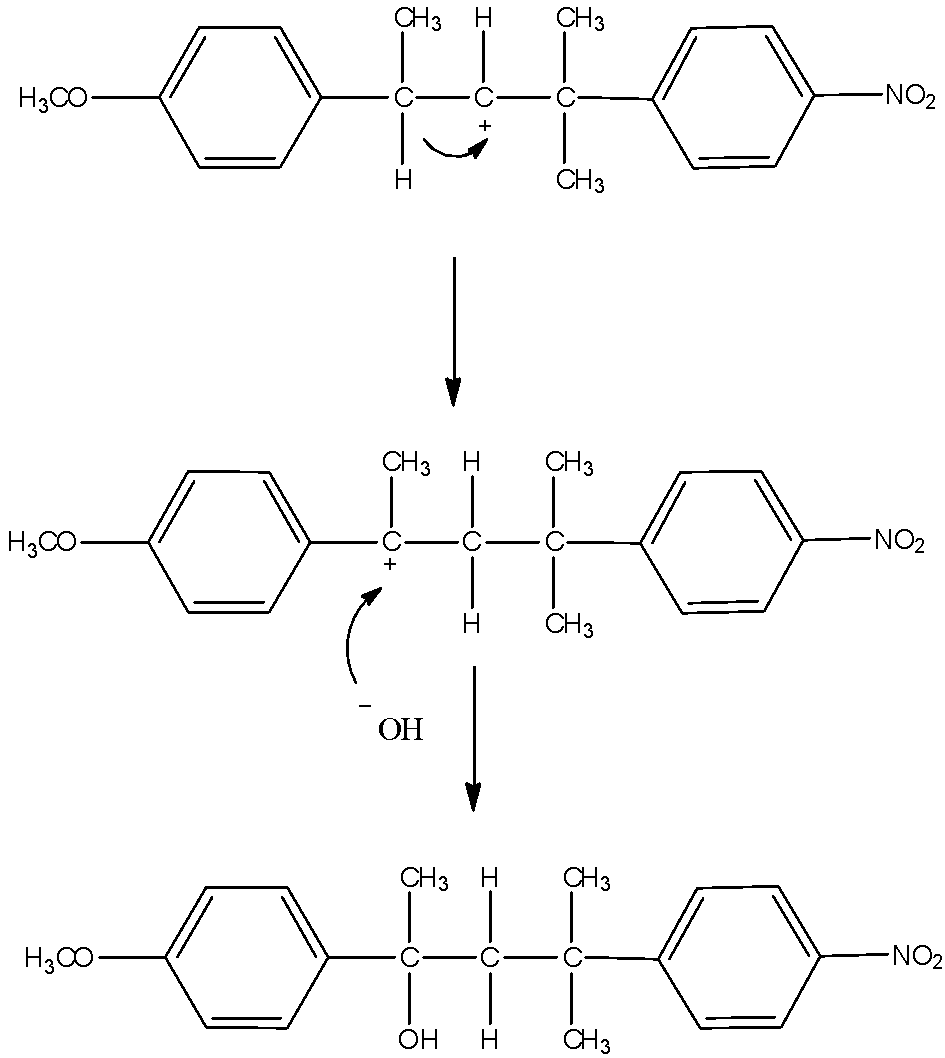
Thus, from the above given reaction, we can say that both products K and L will be formed.
So, the correct answer is (A).
Note:
In this reaction, hydride shift occurs here because the other possibility is only methyl shift. Methyl shift is not favorable in comparison with hydride shift. If we compare the ratio of the products formed, then I will be formed more because the carbocation formed in this reaction is more stable as it is a benzylic carbocation which gets stabilized by resonance also.
Complete answer:
We are given a compound and we need to find the product of its aqueous hydrolysis. The solvent medium is of aqueous acetone here.
- From the structure of the compound, we can say that ether group and nitro group will be stable and will not react with aqueous acetone. The chlorine atom will be a reactive site in the compound.
- As the reaction medium contains water which is a polar solvent and the chlorine bearing carbon is a secondary carbon, both will favor ${S_N}1$ type of reaction. So, chlorine will leave and form a carbocation. This carbocation will be attacked by the nucleophile $H{O^ - }$ of the water. This will substitute –OH group in place of –Cl. The mechanism can be given as:

Now, another possibility is also there. The carbocation formed can undergo hydride shift to make different carbocation. That will give rise to another product. Here, the possibility is that either hydrogen atom shifts of methyl group shifts. Hydrogen atoms always favor migration in comparison with methyl groups. So, that and the formation of new more stable benzylic carbocation is the reason why hydride shift occurs here. The mechanism of the formation of another product is here.

Thus, from the above given reaction, we can say that both products K and L will be formed.
So, the correct answer is (A).
Note:
In this reaction, hydride shift occurs here because the other possibility is only methyl shift. Methyl shift is not favorable in comparison with hydride shift. If we compare the ratio of the products formed, then I will be formed more because the carbocation formed in this reaction is more stable as it is a benzylic carbocation which gets stabilized by resonance also.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




