
Hydrolysis of 1 mol of peroxodisulphuric acid produces:
A. 2 mol of sulphuric acid.
B. 2 mol of peroxomo sulphuric acid
C. 1 mol of sulphuric acid and 1 mole of peroxomo sulphuric acid
D. 1 mol of sulphuric acid, 1 mole of peroxomo sulphuric acid and 1 mol of hydrogen peroxide
Answer
562.8k+ views
Hint: THydrolysis is a reaction where addition of water takes place. On hydrolysis of one mol of Peroxodisulphuric acid, water molecules break the peroxodisulphuric acid into two new products.
Complete step by step answer:
Hydrolysis is a chemical reaction where water is used as one of the reactants to break the chemical bond present between the atoms of the other reactant molecule.
Peroxodisulphuric acid is an inorganic compound which has a chemical formula of \[{H_2}{S_2}{O_8}\]. It is also named as Marshall’s acid which was named after its inventor Professor Hugh Marshall. It is a Sulphur oxoacid which is used as an oxidizing agent. In Peroxodisulphuric acid, the Sulphur molecule is present in +6 oxidation state and a peroxide group.
On hydrolysis of 1 mol of Peroxodisulphuric acid, water molecules break the peroxodisulphuric acid into two new products.
The reaction for the hydrolysis of 1 mol of Peroxodisulphuric acid is shown below.
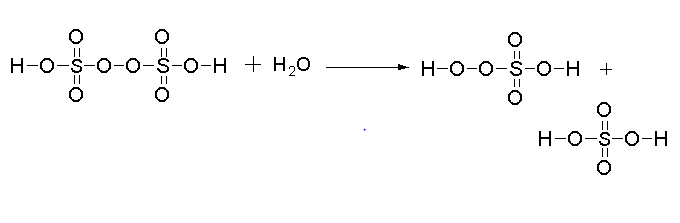
In this reaction one mole of peroxodisulphuric acid reacts with one mole of water to give one mole of peroxomo sulphuric acid and one mole of sulphuric acid.
Therefore, the correct option is C.
Note: The peroxodisulphuric acid is formed by reacting chlorosulfuric acid with hydrogen peroxide where hydrochloric acid is formed as the side product.
Complete step by step answer:
Hydrolysis is a chemical reaction where water is used as one of the reactants to break the chemical bond present between the atoms of the other reactant molecule.
Peroxodisulphuric acid is an inorganic compound which has a chemical formula of \[{H_2}{S_2}{O_8}\]. It is also named as Marshall’s acid which was named after its inventor Professor Hugh Marshall. It is a Sulphur oxoacid which is used as an oxidizing agent. In Peroxodisulphuric acid, the Sulphur molecule is present in +6 oxidation state and a peroxide group.
On hydrolysis of 1 mol of Peroxodisulphuric acid, water molecules break the peroxodisulphuric acid into two new products.
The reaction for the hydrolysis of 1 mol of Peroxodisulphuric acid is shown below.

In this reaction one mole of peroxodisulphuric acid reacts with one mole of water to give one mole of peroxomo sulphuric acid and one mole of sulphuric acid.
Therefore, the correct option is C.
Note: The peroxodisulphuric acid is formed by reacting chlorosulfuric acid with hydrogen peroxide where hydrochloric acid is formed as the side product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




