
How many hybrid orbitals are there in ${\text{HN}}{{\text{O}}_{\text{3}}}$?
Answer
574.2k+ views
Hint For the calculation of the number of hybrid orbitals present in the molecule, firstly we have to know about the structure of that molecule then we will calculate hybrid orbitals by knowing hybridization of each atom present in it.
Complete step by step solution: As we know, hybrid orbitals are formed by the combination of atomic orbitals and geometry of this formed hybrid orbitals are always different from the parent atomic orbitals.
First we draw the structure of ${\text{HN}}{{\text{O}}_{\text{3}}}$ and its structure is the hybrid of two resonance structures, which are shown as follow:
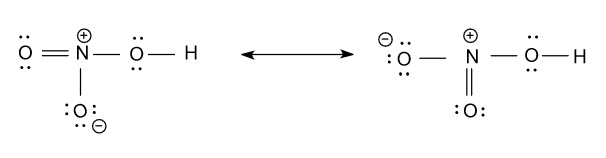
Now we calculate the hybridization of each atom present in the above molecule as follow:
-Hybridization of nitrogen atom (${\text{N}}$) in the above figure is ${\text{s}}{{\text{p}}^{\text{2}}}$i.e. three hybrid orbitals are present in nitrogen, as it has three sigma bonds connected to it.
-Hybridization of the oxygen atom (${\text{O}}$) atom which is bonded with hydrogen atom (${\text{H}}$) atom is ${\text{s}}{{\text{p}}^{\text{3}}}$i.e. in this oxygen atom four hybrid orbitals are present as it has two sigma bonds and two lone pairs of electron in it.
-Out of the remaining two oxygen atoms (${\text{O}}$), hybridization of one oxygen atom (${\text{O}}$) is ${\text{s}}{{\text{p}}^{\text{2}}}$i.e. three hybrid orbitals are present, as it has one sigma bond and two lone pairs of electron in it. And the hybridization of another oxygen atom (${\text{O}}$) is ${\text{s}}{{\text{p}}^{\text{3}}}$i.e. in this oxygen atom four hybrid orbitals are present as it has one sigma bond and three lone pairs of electron in it.
-But the above two oxygen atoms are showing resonance in the Lewis dot structure i.e. these two oxygen are equivalent to each other are showing same hybridization which is ${\text{s}}{{\text{p}}^{\text{2}}}$, it means they have three hybrid orbitals in it.
-Now we add hybrid orbitals of each atom present in the molecule for calculating the total number of hybrid orbitals in ${\text{HN}}{{\text{O}}_{\text{3}}}$.
So, hybrid orbitals in ${\text{HN}}{{\text{O}}_{\text{3}}}$ = Hybrid orbitals of nitrogen + Hybrid orbitals of oxygen
Hybrid orbitals in ${\text{HN}}{{\text{O}}_{\text{3}}}$ = $3 + 4 + 3 + 3 = 13$.
Hence, $13$ hybrid orbitals are there in ${\text{HN}}{{\text{O}}_{\text{3}}}$.
Note: Here some of you may think that why we didn’t calculate the number of hybrid orbitals of hydrogen atom, so the reason is that hydrogen atom is connected with only one sigma bond which will not show any type of hybridization.
Complete step by step solution: As we know, hybrid orbitals are formed by the combination of atomic orbitals and geometry of this formed hybrid orbitals are always different from the parent atomic orbitals.
First we draw the structure of ${\text{HN}}{{\text{O}}_{\text{3}}}$ and its structure is the hybrid of two resonance structures, which are shown as follow:

Now we calculate the hybridization of each atom present in the above molecule as follow:
-Hybridization of nitrogen atom (${\text{N}}$) in the above figure is ${\text{s}}{{\text{p}}^{\text{2}}}$i.e. three hybrid orbitals are present in nitrogen, as it has three sigma bonds connected to it.
-Hybridization of the oxygen atom (${\text{O}}$) atom which is bonded with hydrogen atom (${\text{H}}$) atom is ${\text{s}}{{\text{p}}^{\text{3}}}$i.e. in this oxygen atom four hybrid orbitals are present as it has two sigma bonds and two lone pairs of electron in it.
-Out of the remaining two oxygen atoms (${\text{O}}$), hybridization of one oxygen atom (${\text{O}}$) is ${\text{s}}{{\text{p}}^{\text{2}}}$i.e. three hybrid orbitals are present, as it has one sigma bond and two lone pairs of electron in it. And the hybridization of another oxygen atom (${\text{O}}$) is ${\text{s}}{{\text{p}}^{\text{3}}}$i.e. in this oxygen atom four hybrid orbitals are present as it has one sigma bond and three lone pairs of electron in it.
-But the above two oxygen atoms are showing resonance in the Lewis dot structure i.e. these two oxygen are equivalent to each other are showing same hybridization which is ${\text{s}}{{\text{p}}^{\text{2}}}$, it means they have three hybrid orbitals in it.
-Now we add hybrid orbitals of each atom present in the molecule for calculating the total number of hybrid orbitals in ${\text{HN}}{{\text{O}}_{\text{3}}}$.
So, hybrid orbitals in ${\text{HN}}{{\text{O}}_{\text{3}}}$ = Hybrid orbitals of nitrogen + Hybrid orbitals of oxygen
Hybrid orbitals in ${\text{HN}}{{\text{O}}_{\text{3}}}$ = $3 + 4 + 3 + 3 = 13$.
Hence, $13$ hybrid orbitals are there in ${\text{HN}}{{\text{O}}_{\text{3}}}$.
Note: Here some of you may think that why we didn’t calculate the number of hybrid orbitals of hydrogen atom, so the reason is that hydrogen atom is connected with only one sigma bond which will not show any type of hybridization.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




