
How will you convert ethyne to ethanal?
Answer
576.9k+ views
Hint: So, let's first convert ethyne to ethene by hydrogenation. During the catalytic hydrogenation of ethyne, ethene is formed first, which is further reduced in the next step to ethane.
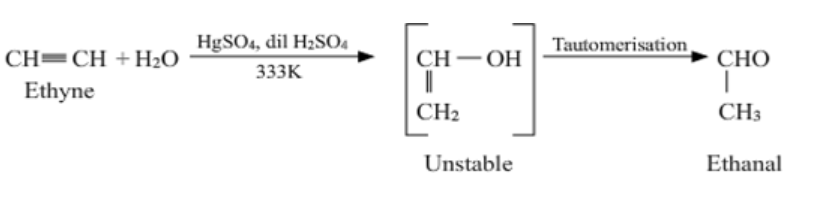
Complete step by step answer: Ethyne on hydration in presence of 40% ${H_2}{SO_4}$ and 1% $HgSO_4$ gives vinyl alcohol, which tautomerize to form acetaldehyde.
Ethanal is practically incompatible with heavy oxidizers, acids , alcohols, ammonia, amines, phenols, ketones, halogens and anhydrides. With anhydrous ammonia, hydrogen cyanide, hydrogen sulphide, and alkaline materials (such as sodium hydroxide), it reacts violently. Sometimes, as it reacts with air, unstable peroxides are produced that can explode.
Additional Information:
-Often referred to as acetaldehyde, ethanal is used. Below are a few major uses of acetaldehyde:
-Ethanal is an intermediate in the synthesis of other chemicals.
-The oxygenated solvent Ethyl Acetate, Pentaerythritol (used in the processing of synthetic resins for the painting industry) and Pyridines are the main derivatives of Ethanal.
-It is used for the processing of fragrances, polyester resins and simple dyes.
-In the rubber, tanning and paper industries, it is often used as a solvent, as a fruit and fish preservative, as a flavouring agent, as a gelatin hardener, as an alcohol denaturant and in the composition of gasoline.
Note: Ethanal, is written as ${CH_3}{CHO}$; methanal as $HCHO$. In the longest chain, the name counts the total number of carbon atoms, including one in the carbonyl group. Note that number 1 is always counted from the carbon atom of the carbonyl group, if you have side groups attached to the chain.
Complete step by step answer: Ethyne on hydration in presence of 40% ${H_2}{SO_4}$ and 1% $HgSO_4$ gives vinyl alcohol, which tautomerize to form acetaldehyde.

Ethanal is practically incompatible with heavy oxidizers, acids , alcohols, ammonia, amines, phenols, ketones, halogens and anhydrides. With anhydrous ammonia, hydrogen cyanide, hydrogen sulphide, and alkaline materials (such as sodium hydroxide), it reacts violently. Sometimes, as it reacts with air, unstable peroxides are produced that can explode.
Additional Information:
-Often referred to as acetaldehyde, ethanal is used. Below are a few major uses of acetaldehyde:
-Ethanal is an intermediate in the synthesis of other chemicals.
-The oxygenated solvent Ethyl Acetate, Pentaerythritol (used in the processing of synthetic resins for the painting industry) and Pyridines are the main derivatives of Ethanal.
-It is used for the processing of fragrances, polyester resins and simple dyes.
-In the rubber, tanning and paper industries, it is often used as a solvent, as a fruit and fish preservative, as a flavouring agent, as a gelatin hardener, as an alcohol denaturant and in the composition of gasoline.
Note: Ethanal, is written as ${CH_3}{CHO}$; methanal as $HCHO$. In the longest chain, the name counts the total number of carbon atoms, including one in the carbonyl group. Note that number 1 is always counted from the carbon atom of the carbonyl group, if you have side groups attached to the chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




