
How do you make ether?
Answer
549k+ views
Hint: Either is an organic compound is ether group ${{\text{(}{{\text{C}}_{2}}{{\text{H}}_{5}}\text{)}}_{\text{2}}}\text{O}$. It is a colorless, highly volatile, sweet smelling, extremely flammable liquid. It is commonly used as a solvent in laboratories and as the starting fluid for some engines.
Complete step by step answer:
The general structure of an ether is $\text{R}\text{O}\text{R}\prime \text{,}$ where $\text{R}$ and $\text{R}\prime $ represent the alkyl or aryl groups.
If alkyl groups are the same on both sides of an oxygen atom, then it is a simple or symmetrical ether whereas if they are different they are called mixed or unsymmetrical ether.
There are two primary reactions to generate ether: either by dehydration of alcohols or by williamson synthesis
Dehydration of alcohols: \[\text{2R}\text{OH}\to \text{R}\text{O}\text{R}+{{\text{H}}_{2}}\text{O}\] at high temperature.
Primary alcohols are converted to ethers on heating is the presence of an acid catalyst, usually sulfuric acid.
E.g. \[\underset{\text{1-Butanol}}{\mathop{\text{C}{{\text{H}}_{3}}\text{CC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{OH}}}\,\ \overset{{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}}{\mathop{\to }}\,\underset{\text{Dibutyl ether}}{\mathop{\text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{OC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{3}}}}\,+\underset{\text{Water}}{\mathop{{{\text{H}}_{2}}\text{O}}}\,\text{.}\]
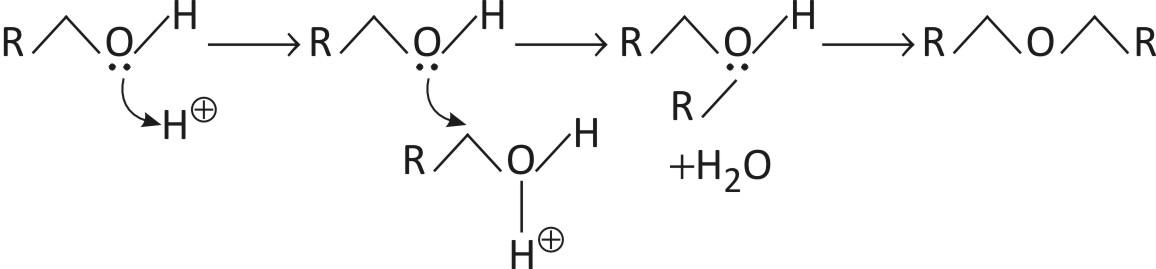
The direct nucleophilic substitution reaction requires elevated temperatures. The reaction is catalysed by acids, usually sulfuric acid. Diethyl ether is produced from ethanol by this method. Cyclic ethers are readily generated by this approach. The dehydration route after requires conditions incompatible with delicate molecules.
Williamson ether synthesis
$\text{R}\text{ONa}+\text{R’} \text{X}\to \text{R}\text{O}\text{R’} +\text{Max}$
This reaction is called williamson ether synthesis. It involves treatment of a parent alcohol with a strong base to form the alpoxide, followed by addition of an appropriate aliphatic compound bearing a suitable learning group $(\text{R}\text{X})$. Suitable having groups \[\text{X-}\] include iodide, bromide or sulfonates. In a related reaction, alkyl halides undergo nucleophilic displacement by phenoxides. The \[\text{R}\text{X}\] cannot be used to react with the alcohol. However, phenols can be used to replace the alcohol while maintaining the alkyl halide. Since phenols are acidic, they readily react with a strong base like sodium hydroxide to form phenoxide ions. The phenoxide ion will then substitute the $\text{X}$-group in the alkyl halide, forming an ether with an aryl group attached to it in a reaction with an \[\text{S}{{\text{N}}_{2}}\] mechanism.
${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}+\text{O}{{\text{H}}^{-}}\to \text{CH}-{{\text{O}}^{-}}+{{\text{H}}_{2}}\text{O}$
${{\text{C}}_{6}}{{\text{H}}_{5}}^{-}{{\text{O}}^{-}}+\text{R}\text{X}\to {{\text{C}}_{6}}{{\text{H}}_{5}}\text{OR}$
Additional Information:
The $\text{C}\text{O}$ bonds that comprise simple ethers are strong. They are unreactive toward all but the strongest bases. Although generally of low chemical reactivity, they are more reactive than alkanes. When stored in the presence of air or oxygen, ehlers tend to form explosive peroxides, such as diethyl ether hydroperoxide. This reaction is accelerated by light, metal catalyst and aldehydes. Ethers are common in organic chemistry and are more prevalent in biochemistry as they are common linkages in carbohydrates. Ethers have boiling points similar to those of the analogous alkanes. Diethyl ether has high volatility and low flush point. So it is also used as a component of the fuel mixture for carbureted compression ignition model engines.
Note: Diethyl ether is anesthetic dosage is an inhalant which has a long history of recreational use. One disadvantage is the high flammability with oxygen. Diethyl ether inhibits alcohol dehydrogenase and slows the metabolism of ethanol. The dangerous properties of ether provides are the reason that diethyl ether and other provides forming ethers like ethylene glycol dimethyl ether are avoided in industrial processes. Ethers resist hydrolysis, they are cleaned by hydrobromic and hydroiodic acid.
Complete step by step answer:
The general structure of an ether is $\text{R}\text{O}\text{R}\prime \text{,}$ where $\text{R}$ and $\text{R}\prime $ represent the alkyl or aryl groups.
If alkyl groups are the same on both sides of an oxygen atom, then it is a simple or symmetrical ether whereas if they are different they are called mixed or unsymmetrical ether.
There are two primary reactions to generate ether: either by dehydration of alcohols or by williamson synthesis
Dehydration of alcohols: \[\text{2R}\text{OH}\to \text{R}\text{O}\text{R}+{{\text{H}}_{2}}\text{O}\] at high temperature.
Primary alcohols are converted to ethers on heating is the presence of an acid catalyst, usually sulfuric acid.
E.g. \[\underset{\text{1-Butanol}}{\mathop{\text{C}{{\text{H}}_{3}}\text{CC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{OH}}}\,\ \overset{{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}}{\mathop{\to }}\,\underset{\text{Dibutyl ether}}{\mathop{\text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{OC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{3}}}}\,+\underset{\text{Water}}{\mathop{{{\text{H}}_{2}}\text{O}}}\,\text{.}\]

The direct nucleophilic substitution reaction requires elevated temperatures. The reaction is catalysed by acids, usually sulfuric acid. Diethyl ether is produced from ethanol by this method. Cyclic ethers are readily generated by this approach. The dehydration route after requires conditions incompatible with delicate molecules.
Williamson ether synthesis
$\text{R}\text{ONa}+\text{R’} \text{X}\to \text{R}\text{O}\text{R’} +\text{Max}$
This reaction is called williamson ether synthesis. It involves treatment of a parent alcohol with a strong base to form the alpoxide, followed by addition of an appropriate aliphatic compound bearing a suitable learning group $(\text{R}\text{X})$. Suitable having groups \[\text{X-}\] include iodide, bromide or sulfonates. In a related reaction, alkyl halides undergo nucleophilic displacement by phenoxides. The \[\text{R}\text{X}\] cannot be used to react with the alcohol. However, phenols can be used to replace the alcohol while maintaining the alkyl halide. Since phenols are acidic, they readily react with a strong base like sodium hydroxide to form phenoxide ions. The phenoxide ion will then substitute the $\text{X}$-group in the alkyl halide, forming an ether with an aryl group attached to it in a reaction with an \[\text{S}{{\text{N}}_{2}}\] mechanism.
${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}+\text{O}{{\text{H}}^{-}}\to \text{CH}-{{\text{O}}^{-}}+{{\text{H}}_{2}}\text{O}$
${{\text{C}}_{6}}{{\text{H}}_{5}}^{-}{{\text{O}}^{-}}+\text{R}\text{X}\to {{\text{C}}_{6}}{{\text{H}}_{5}}\text{OR}$
Additional Information:
The $\text{C}\text{O}$ bonds that comprise simple ethers are strong. They are unreactive toward all but the strongest bases. Although generally of low chemical reactivity, they are more reactive than alkanes. When stored in the presence of air or oxygen, ehlers tend to form explosive peroxides, such as diethyl ether hydroperoxide. This reaction is accelerated by light, metal catalyst and aldehydes. Ethers are common in organic chemistry and are more prevalent in biochemistry as they are common linkages in carbohydrates. Ethers have boiling points similar to those of the analogous alkanes. Diethyl ether has high volatility and low flush point. So it is also used as a component of the fuel mixture for carbureted compression ignition model engines.
Note: Diethyl ether is anesthetic dosage is an inhalant which has a long history of recreational use. One disadvantage is the high flammability with oxygen. Diethyl ether inhibits alcohol dehydrogenase and slows the metabolism of ethanol. The dangerous properties of ether provides are the reason that diethyl ether and other provides forming ethers like ethylene glycol dimethyl ether are avoided in industrial processes. Ethers resist hydrolysis, they are cleaned by hydrobromic and hydroiodic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




