
How do you make acetic anhydride?
Answer
546.6k+ views
Hint:Acetic anhydride is an organic compound containing an anhydride functional group. The molecular formula of the acetic anhydride is \[{{\text{C}}_4}{{\text{H}}_6}{{\text{O}}_3}\].
It is a hazardous compound hence, not safe to use. It is highly corrosive, irritates skin and eyes.
At high concentration, it also damages the lungs and also affects the nose, throat, mounth. Hence, there is a need to handle it carefully.
Complete step-by-step answer:
Acetic anhydride possesses chemical formula is \[{{\text{C}}_4}{{\text{H}}_6}{{\text{O}}_3}\] and its structure is as follows:
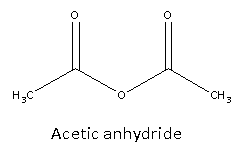
From structure, we can say that it is symmetric.
It is prepared from the acyl chloride and sodium salt of the acetic acid and the reaction is as follows:
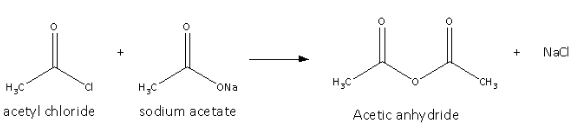
Here, we can see that the chlorine from the acetyl chloride and sodium from the sodium salt of acetic acid form sodium chloride.
Other methods are also used to prepare the acetic anhydride. Acetic anhydride is also prepared by the dehydration of the acetic acid at \[800^\circ {\text{C}}\]. It also can be prepared by the carbonylation of methyl acetate.
Note: Based on functional groups present in the organic compounds different types are acids, alcohols, anhydrides, halides, ketones, aldehydes, etc.
Acetic anhydride belongs to the anhydride functional group.
Acetic anhydride is used as raw material for the synthesis of plastic and cellulose acetate fibers. It is also used as a raw material in pharmaceuticals for the synthesis of aspirin, acetaminophen, etc.
In chemical synthesis, it is widely used as an acetylating agent which causes acetylation of the material.
It affects the different parts of the body like skin, lungs, mouth, nose, throat, etc hence, there is a need to handle it carefully.
It is a hazardous compound hence, not safe to use. It is highly corrosive, irritates skin and eyes.
At high concentration, it also damages the lungs and also affects the nose, throat, mounth. Hence, there is a need to handle it carefully.
Complete step-by-step answer:
Acetic anhydride possesses chemical formula is \[{{\text{C}}_4}{{\text{H}}_6}{{\text{O}}_3}\] and its structure is as follows:

From structure, we can say that it is symmetric.
It is prepared from the acyl chloride and sodium salt of the acetic acid and the reaction is as follows:

Here, we can see that the chlorine from the acetyl chloride and sodium from the sodium salt of acetic acid form sodium chloride.
Other methods are also used to prepare the acetic anhydride. Acetic anhydride is also prepared by the dehydration of the acetic acid at \[800^\circ {\text{C}}\]. It also can be prepared by the carbonylation of methyl acetate.
Note: Based on functional groups present in the organic compounds different types are acids, alcohols, anhydrides, halides, ketones, aldehydes, etc.
Acetic anhydride belongs to the anhydride functional group.
Acetic anhydride is used as raw material for the synthesis of plastic and cellulose acetate fibers. It is also used as a raw material in pharmaceuticals for the synthesis of aspirin, acetaminophen, etc.
In chemical synthesis, it is widely used as an acetylating agent which causes acetylation of the material.
It affects the different parts of the body like skin, lungs, mouth, nose, throat, etc hence, there is a need to handle it carefully.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




