
Homogeneous mixtures are also called:
A. pure substances
B. elements
C. solids
D. compounds
E. solutions
Answer
603.3k+ views
Hint: ‘homo’ – same
‘hetero’ – different
A homogeneous mixture is a solid, liquid or gaseous mixture that has an equal proportion of components in a medium. It may exist in any phase, and it consists of solute and solvent. The particles are not visible through the naked eye.
Complete step by step answer:
Homogeneous mixtures are also called solutions.
A solution is a homogeneous mixture of two or more substances.
A solute is a component which gets dissolved in a medium. The solvent is the medium in which solute dissolves.
For example, in saline solution salt is the solute dissolved in water as a solvent.
Solute has lower concentration whereas solvent has a higher concentration in solution.
A solution does not scatter light beams and components cannot be separated using simple mechanical filtration.
It can exist in any phase (solid, liquid, gas)
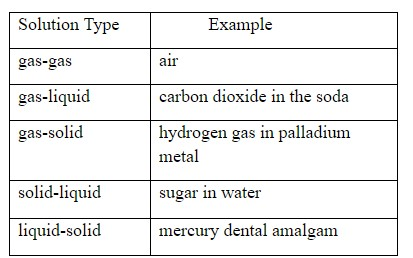
So, the correct option is E.
Additional information:
All solutions are considered as homogeneous because the dissolved material is present in the same composition throughout the solution.
Note: The components of a mixture can be separated easily whereas that of solution cannot be. A solution is always clear, do not settle down with time and can pass through filter paper.
‘hetero’ – different
A homogeneous mixture is a solid, liquid or gaseous mixture that has an equal proportion of components in a medium. It may exist in any phase, and it consists of solute and solvent. The particles are not visible through the naked eye.
Complete step by step answer:
Homogeneous mixtures are also called solutions.
A solution is a homogeneous mixture of two or more substances.
A solute is a component which gets dissolved in a medium. The solvent is the medium in which solute dissolves.
For example, in saline solution salt is the solute dissolved in water as a solvent.
Solute has lower concentration whereas solvent has a higher concentration in solution.
A solution does not scatter light beams and components cannot be separated using simple mechanical filtration.
It can exist in any phase (solid, liquid, gas)

So, the correct option is E.
Additional information:
All solutions are considered as homogeneous because the dissolved material is present in the same composition throughout the solution.
Note: The components of a mixture can be separated easily whereas that of solution cannot be. A solution is always clear, do not settle down with time and can pass through filter paper.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




