
Haloarenes undergo Wurtz-Fittig reaction.
(i) What is the Wurtz-Fittig reaction?
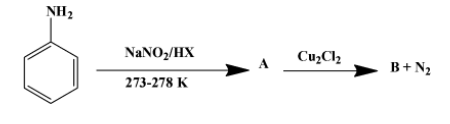
(ii) Write the formula of A and B in the reaction given below-

Answer
595.2k+ views
Hint:
(i) It is a reaction between an aliphatic and an aromatic compound with a metal as a reagent inside a medium of dry ether. The product formed is a coupled compound between the reactants.
(ii) A is a form of diazonium salt. B is an aromatic halide.
Complete step by step answer:
(i) The Wurtz-Fittig reaction is a coupling reaction between an aliphatic halide and aromatic halide. The sodium metal is a reagent used to facilitate the reaction in a medium of dry ether. The medium is “dry” because any moisture content could intervene in the reaction giving unwanted by-products. The reaction is as follows:
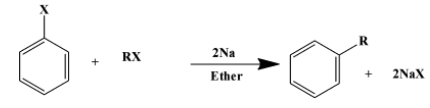 (here “X” is $Cl$ or $Br$)
(here “X” is $Cl$ or $Br$)
But this reaction also produces many by-products which are not always desirable. This is because coupling can also take place between two or more aliphatic compounds or two aromatic compounds. The former is called Wurtz reaction and the latter is called Fittig reaction. This happens because these reactions proceed via a free-radical mechanism and therefore do not have specificity. Their combination can be very random and therefore controlled environments are used to obtain required products. We see the general formula of the two reactions discussed here below:
Wurtz reaction
\[2RX+2Na\xrightarrow{Ether}R-R+2NaX\]
Fittig reaction
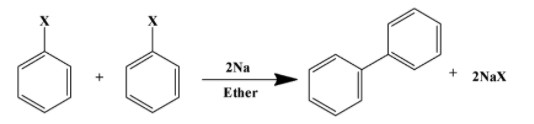
ii)
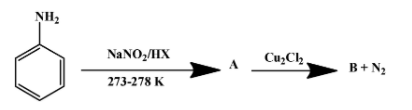
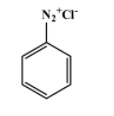
The reagents used in the first reaction are $NaN{{O}_{2}}/HX$ at $\text{(273-278)K}$. They convert aniline into diazonium salt. That implies A is the diazonium salt as shown below-
The second reaction is one of the several ones that can be done with a diazonium salt. In this particular case, ${{N}_{2}}^{+}C{{l}^{-}}$ group is replaced by chloride molecule forming chlorobenzene with nitrogen gas as a by-product. The reaction is as follow:
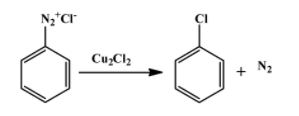
Therefore, product B is chlorobenzene. The above reaction proceeds via two steps. First the diazonium molecule becomes a free radical when$C{{u}^{+}}$ion donates one electron to it. The chloride ion then behaves as a nucleophile to attack the free radical formed in the first step.
Note:
i) The Wurtz-Fittig reaction is different from Wurtz reaction and Fittig reaction as mentioned above. Only symmetric compounds should be synthesized using this method.
ii) The diazonium is a reactive salt which can readily undergo homolytic cleavage to produce free radicals. This is convenient for chemists because they can utilise this to form many
different products by giving an appropriate reagent that can react via producing a nucleophile.
(i) It is a reaction between an aliphatic and an aromatic compound with a metal as a reagent inside a medium of dry ether. The product formed is a coupled compound between the reactants.
(ii) A is a form of diazonium salt. B is an aromatic halide.
Complete step by step answer:
(i) The Wurtz-Fittig reaction is a coupling reaction between an aliphatic halide and aromatic halide. The sodium metal is a reagent used to facilitate the reaction in a medium of dry ether. The medium is “dry” because any moisture content could intervene in the reaction giving unwanted by-products. The reaction is as follows:

But this reaction also produces many by-products which are not always desirable. This is because coupling can also take place between two or more aliphatic compounds or two aromatic compounds. The former is called Wurtz reaction and the latter is called Fittig reaction. This happens because these reactions proceed via a free-radical mechanism and therefore do not have specificity. Their combination can be very random and therefore controlled environments are used to obtain required products. We see the general formula of the two reactions discussed here below:
Wurtz reaction
\[2RX+2Na\xrightarrow{Ether}R-R+2NaX\]
Fittig reaction

ii)

The reagents used in the first reaction are $NaN{{O}_{2}}/HX$ at $\text{(273-278)K}$. They convert aniline into diazonium salt. That implies A is the diazonium salt as shown below-

The second reaction is one of the several ones that can be done with a diazonium salt. In this particular case, ${{N}_{2}}^{+}C{{l}^{-}}$ group is replaced by chloride molecule forming chlorobenzene with nitrogen gas as a by-product. The reaction is as follow:

Therefore, product B is chlorobenzene. The above reaction proceeds via two steps. First the diazonium molecule becomes a free radical when$C{{u}^{+}}$ion donates one electron to it. The chloride ion then behaves as a nucleophile to attack the free radical formed in the first step.
Note:
i) The Wurtz-Fittig reaction is different from Wurtz reaction and Fittig reaction as mentioned above. Only symmetric compounds should be synthesized using this method.
ii) The diazonium is a reactive salt which can readily undergo homolytic cleavage to produce free radicals. This is convenient for chemists because they can utilise this to form many
different products by giving an appropriate reagent that can react via producing a nucleophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




