
\[{{H}_{3}}P{{O}_{3}}\] is a
A.Dibasic acid
B.Monobasic acid
C.Tribasic acid
D.None of these
Answer
591.9k+ views
Hint: Basicity of acids is going to depend on the number of hydrogen atoms they are going to donate.
Monobasic acids are the acids which comprise one replaceable hydrogen ion per molecule or can donate one hydrogen atom when completely ionized in an aqueous solution.
From example acetic acid contains one replaceable hydrogen ion that means acetic acid (\[C{{H}_{3}}COOH\]) is Monobasic because it ionizes in aqueous solution and releases one hydrogen ion.
Complete answer:
In the question the given chemical is Phosphorous acid (\[{{H}_{3}}P{{O}_{3}}\]).
We have to find the given chemical is monobasic or dibasic or tribasic acid.
To know about the acidic character of the given molecule we should know the structure of the molecule.
The structure of the phosphorous acid is as follows.
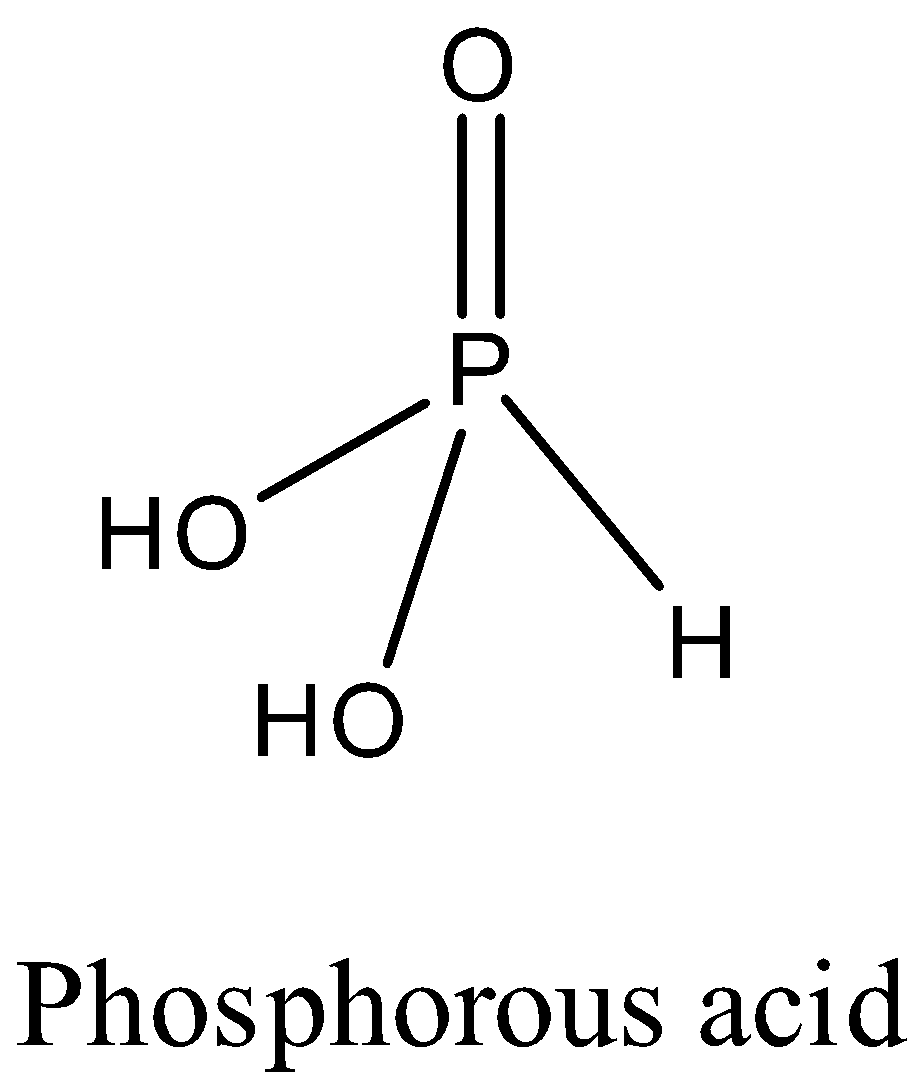
In the above structure two hydrogen atoms are attached to oxygen and one hydrogen atom is attached to phosphorus directly.
The hydrogen atoms attached to oxygen are easily released into an aqueous solution because of high electronegativity of oxygen.
We know that oxygen can take electrons very easily from hydrogen.
So, the two hydrogens attached to oxygen are easily released or donated by the phosphorous acid.
The hydrogen atom which is directly attached to phosphorus atom is not going to be released or donated from phosphorus acid.
Therefore, two hydrogen’s are donated by phosphorus acid.
So, the correct option is A, phosphorus acid is dibasic acid.
Note: In case of hydroxides of phosphorus the acidity is going to depend on the number of hydrogen atoms attached to oxygen atom not on the hydrogen’s which are attached to phosphorus atom.
Monobasic acids are the acids which comprise one replaceable hydrogen ion per molecule or can donate one hydrogen atom when completely ionized in an aqueous solution.
From example acetic acid contains one replaceable hydrogen ion that means acetic acid (\[C{{H}_{3}}COOH\]) is Monobasic because it ionizes in aqueous solution and releases one hydrogen ion.
Complete answer:
In the question the given chemical is Phosphorous acid (\[{{H}_{3}}P{{O}_{3}}\]).
We have to find the given chemical is monobasic or dibasic or tribasic acid.
To know about the acidic character of the given molecule we should know the structure of the molecule.
The structure of the phosphorous acid is as follows.

In the above structure two hydrogen atoms are attached to oxygen and one hydrogen atom is attached to phosphorus directly.
The hydrogen atoms attached to oxygen are easily released into an aqueous solution because of high electronegativity of oxygen.
We know that oxygen can take electrons very easily from hydrogen.
So, the two hydrogens attached to oxygen are easily released or donated by the phosphorous acid.
The hydrogen atom which is directly attached to phosphorus atom is not going to be released or donated from phosphorus acid.
Therefore, two hydrogen’s are donated by phosphorus acid.
So, the correct option is A, phosphorus acid is dibasic acid.
Note: In case of hydroxides of phosphorus the acidity is going to depend on the number of hydrogen atoms attached to oxygen atom not on the hydrogen’s which are attached to phosphorus atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




