
${{H}_{3}}P{{O}_{2}}$is a weak monobasic acid. State whether this statement is True or False.
Answer
554.4k+ views
Hint: Basicity of acids depends on the hydrogen atom, that it is going to donate. When we talk of hydrogen atoms, we refer to those hydrogen atoms which are attached with oxygen. So, to answer this question, we just need to recall the structure of the given acid and count the number of hydrogen atoms attached with oxygen.
Complete step-by-step answer:
In the question, we are provided with Hypophosphorous acid ${{H}_{3}}P{{O}_{2}}$and we are asked to state whether it is a weak monobasic acid or not.
To know the basicity of the given acid we should recall the structure of the given compound.
The structure of hypophosphorous acid is represented below:
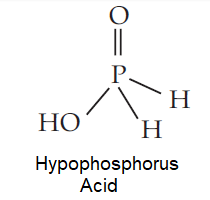
In the above structure we can clearly see that two hydrogen atoms are attached directly to phosphorus and only one hydrogen atom is attached with oxygen.
Now, we know that oxygen is an electronegative element and as a result it can take electrons easily from the hydrogen, thus making it easy for the hydrogen to release easily.
In short, we can say that the hydrogen which is attached directly with oxygen is ionizable and will account for basicity. Since, only one such hydrogen is present in the above structure which is bonded with oxygen. Hence, it will only be released.
Thus, we can interpret the basicity of ${{H}_{3}}P{{O}_{2}}$ to be $1$. Therefore, it is monobasic acid.
We should also note that the more ionizable hydrogen a molecule has the more is its basicity. Here, hypophosphorous acid only has one ionizable hydrogen (hydrogen attached directly to the oxygen in this case). Hence, it is a weak monobasic acid.
Therefore, the given statement in question is true.
Note:As a golden rule it should be noted that in case of hydroxides of phosphorus the basicity will depend on the number of hydrogen atoms attached to the oxygen atom and not on the hydrogen atom which are attached to the phosphorus atoms directly. And the more ionizable hydrogen a molecule has the more is its basicity.
Complete step-by-step answer:
In the question, we are provided with Hypophosphorous acid ${{H}_{3}}P{{O}_{2}}$and we are asked to state whether it is a weak monobasic acid or not.
To know the basicity of the given acid we should recall the structure of the given compound.
The structure of hypophosphorous acid is represented below:

In the above structure we can clearly see that two hydrogen atoms are attached directly to phosphorus and only one hydrogen atom is attached with oxygen.
Now, we know that oxygen is an electronegative element and as a result it can take electrons easily from the hydrogen, thus making it easy for the hydrogen to release easily.
In short, we can say that the hydrogen which is attached directly with oxygen is ionizable and will account for basicity. Since, only one such hydrogen is present in the above structure which is bonded with oxygen. Hence, it will only be released.
Thus, we can interpret the basicity of ${{H}_{3}}P{{O}_{2}}$ to be $1$. Therefore, it is monobasic acid.
We should also note that the more ionizable hydrogen a molecule has the more is its basicity. Here, hypophosphorous acid only has one ionizable hydrogen (hydrogen attached directly to the oxygen in this case). Hence, it is a weak monobasic acid.
Therefore, the given statement in question is true.
Note:As a golden rule it should be noted that in case of hydroxides of phosphorus the basicity will depend on the number of hydrogen atoms attached to the oxygen atom and not on the hydrogen atom which are attached to the phosphorus atoms directly. And the more ionizable hydrogen a molecule has the more is its basicity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




