
Glycosidic linkage is present in:
A. Sucrose
B. Starch
C. Glucose
D. Fructose
Answer
565.5k+ views
Hint: A glycosidic linkage is a type of covalent bond which binds a carbohydrate molecule to another group which may or may not be a carbohydrate. Or simply we can say that glycosidic linkage is one which connects monosaccharides or longer carbohydrate chains to other carbohydrates and forms disaccharides, oligosaccharides and polysaccharides.
Complete step by step answer:
Simply a glycosidic linkage is a covalent bond that holds together a glycoside. A glycoside is a ring shaped sugar molecule that is attached to another molecule. A glycosidic bond formed by the condensation reaction, means one water molecule is produced during the formation of a glycoside. That means the condensation reaction occurs when an alcohol group or ${\text{OH}}$ from a molecule attacks the anomeric carbon of a sugar molecule. Where the anomeric carbon ( carbon has a single bond to two oxygen atoms ) is the central carbon of a hemiacetal.
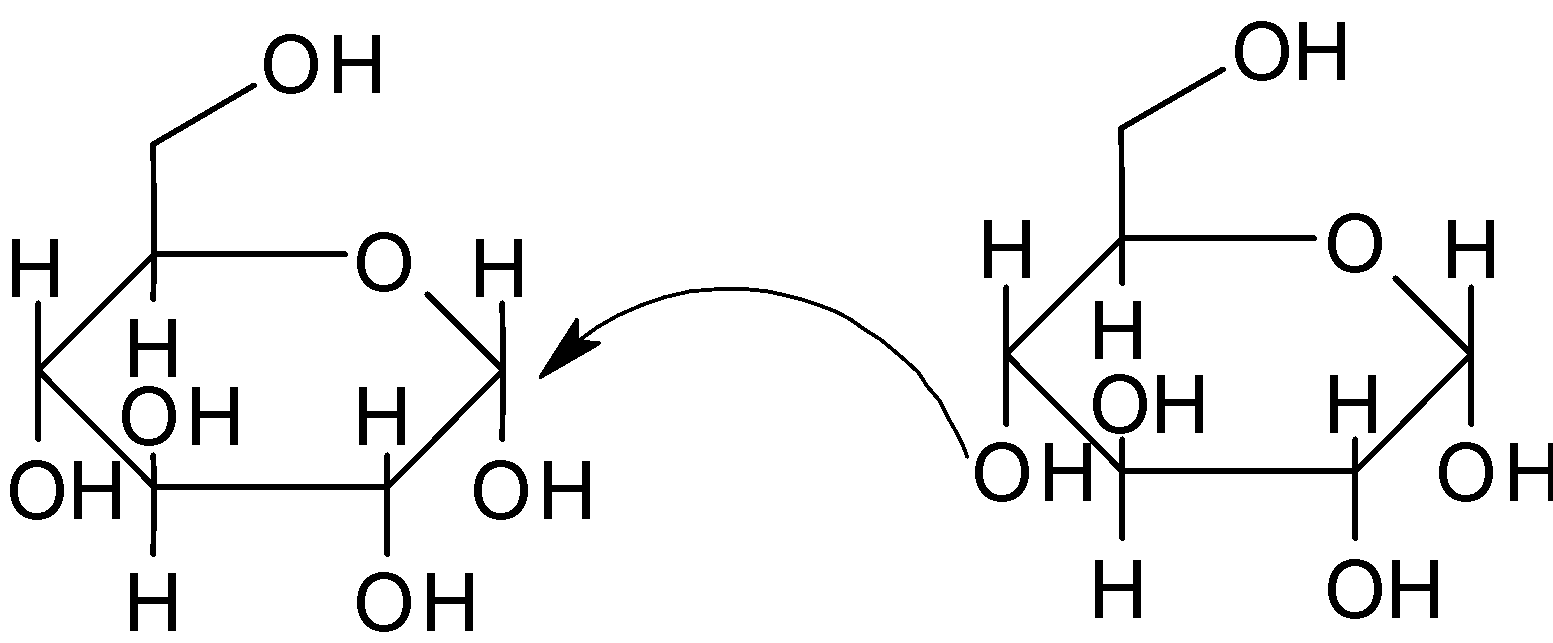
When alcohol group of one sugar molecule attacks the anomeric carbon of other sugar molecule , the ${\text{OH}}$ molecule attached to the anomeric carbon is removed along with the ${\text{H}}$ of the alcohol. That is both ${\text{OH}}$ and ${\text{H}}$ are removed from the original molecule during the reaction and form glycosidic bonds.
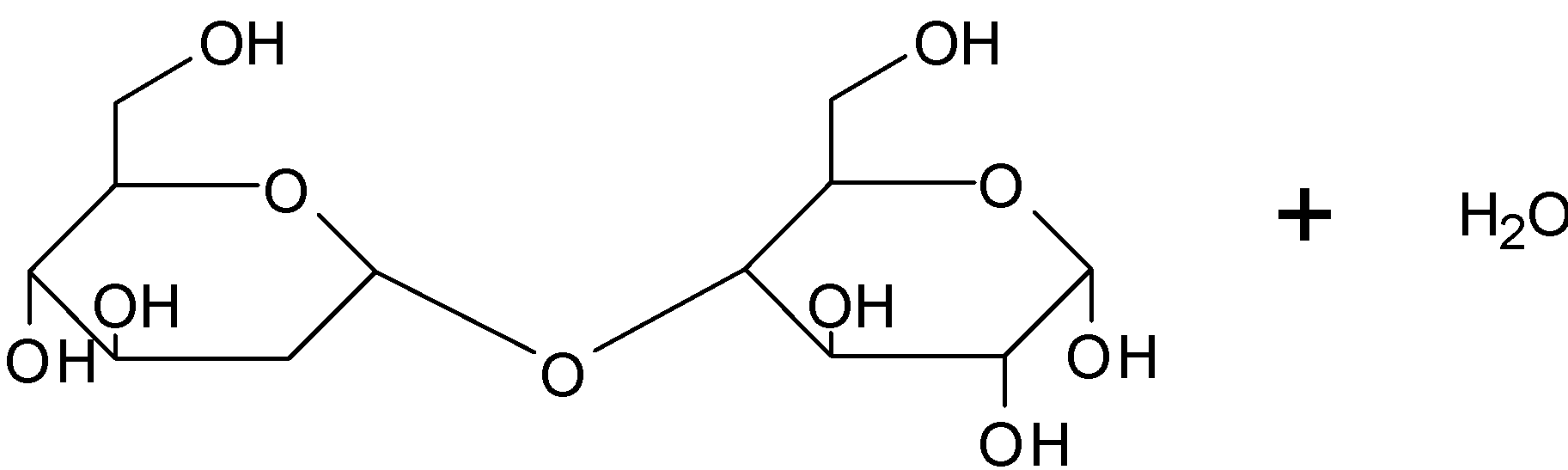
We already know that Carbohydrates are mainly classified into monosaccharides, oligosaccharides and polysaccharides. Where monosaccharides are simple carbohydrates. Both glucose and fructose belong to this group.
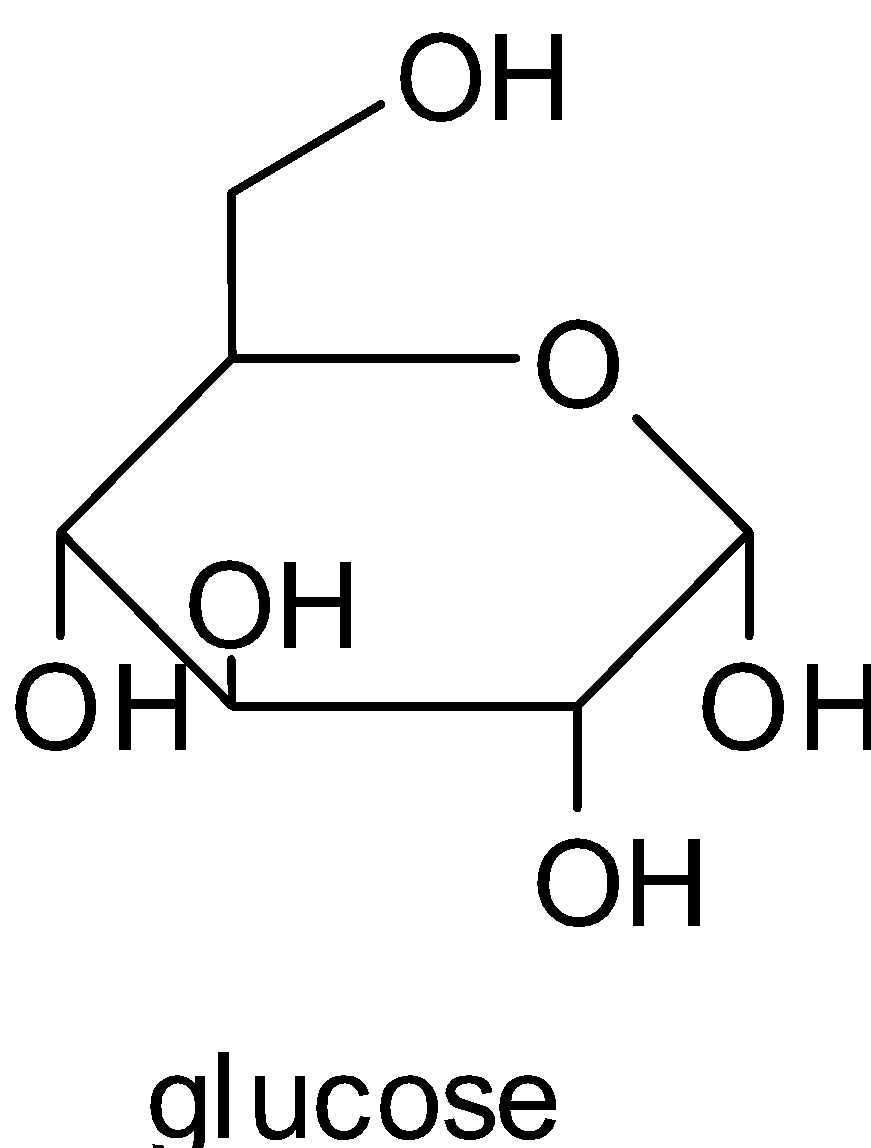
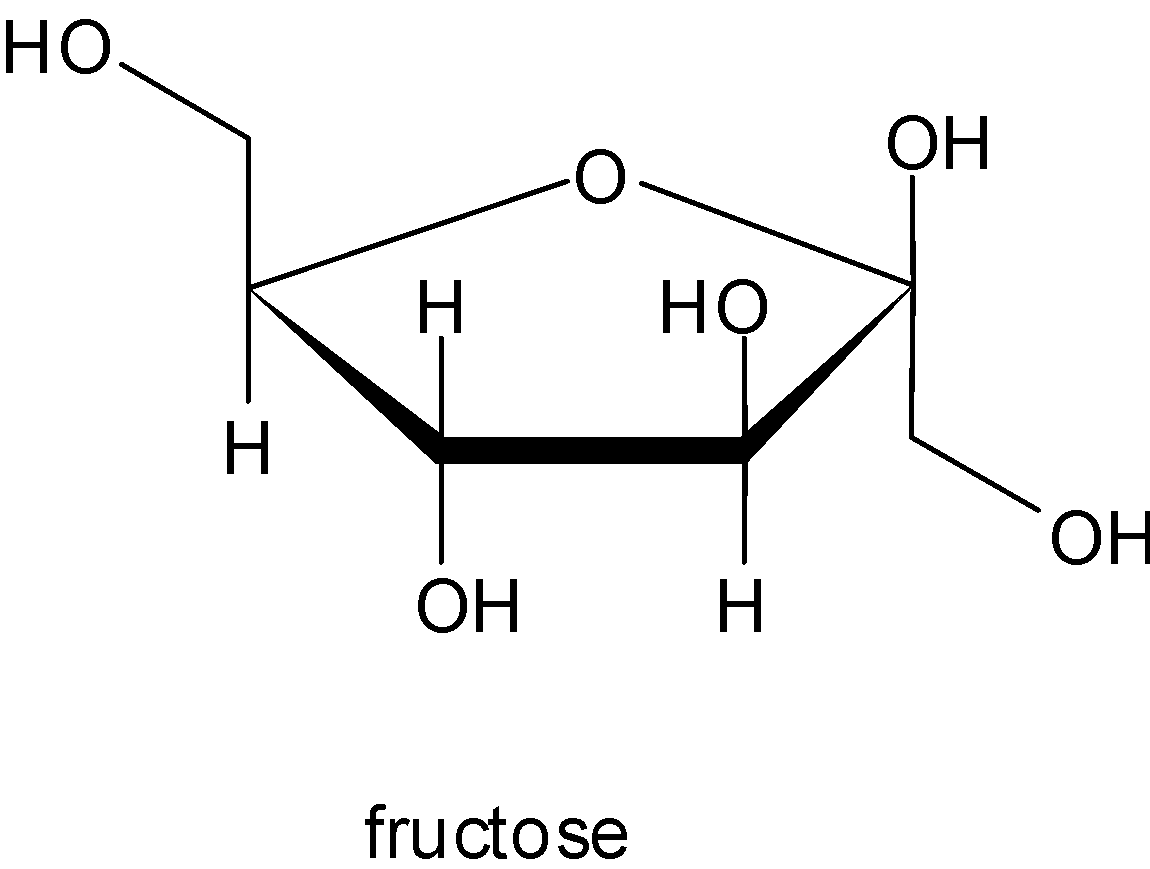
For both glucose and fructose there is no glycosidic linkage as it is clear from the structures.
Oligosaccharides are carbohydrates chains containing two to ten sugar units. In which the monosaccharides are joined together by glycosidic linkage. Sucrose comes under this group. And its structure is given by
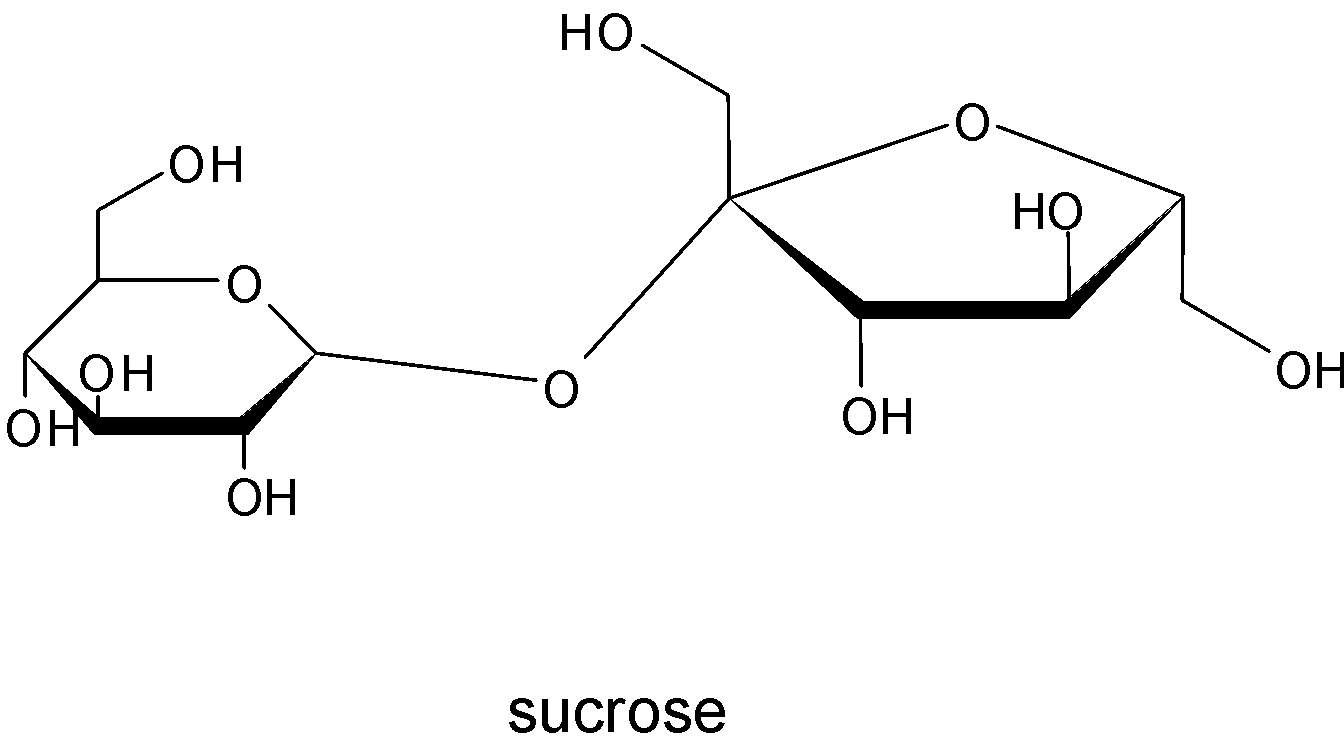
Sucrose is composed of two monosaccharides glucose and fructose , where the two monosaccharides are joined by the glycosidic linkage.
Next is starch, which comes under the polysaccharides group. Where polysaccharides are the long chain of monosaccharides that are linked by glycosidic linkage.
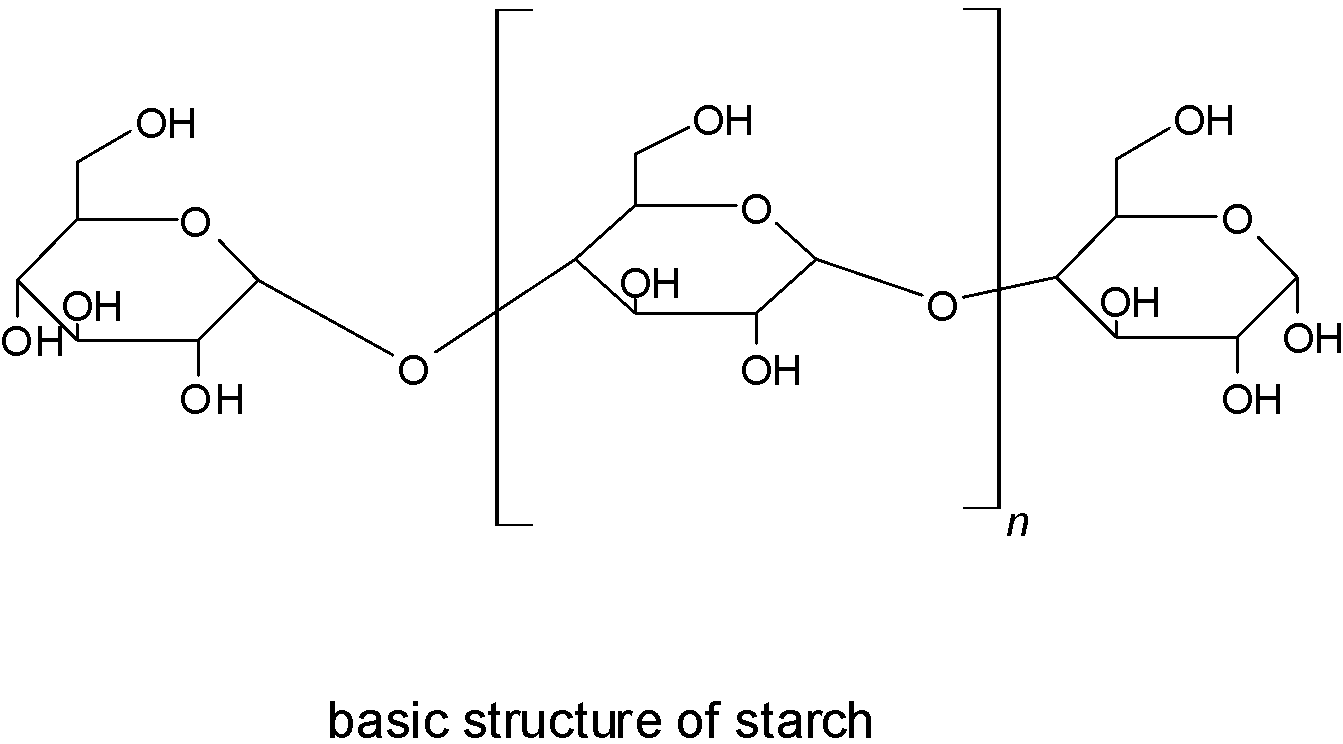
So it is clear that in sucrose and starch glycosidic linkage is present.
so, the correct answer is option A,B..
Note: glycosidic bonds come in different forms and they can be named according to the number of carbon atoms of the sugar molecule which are involved in the linkage. For example 1,4-glycosidic bonds , in which number one carbon atom of one sugar molecule and number fourth carbon atom of other sugar molecule participate in bond formation. There is also a 1,6-glycosidic bond , where again the number one carbon atom of sugar molecule and sixth carbon atom of other sugar molecule is involved.
Complete step by step answer:
Simply a glycosidic linkage is a covalent bond that holds together a glycoside. A glycoside is a ring shaped sugar molecule that is attached to another molecule. A glycosidic bond formed by the condensation reaction, means one water molecule is produced during the formation of a glycoside. That means the condensation reaction occurs when an alcohol group or ${\text{OH}}$ from a molecule attacks the anomeric carbon of a sugar molecule. Where the anomeric carbon ( carbon has a single bond to two oxygen atoms ) is the central carbon of a hemiacetal.

When alcohol group of one sugar molecule attacks the anomeric carbon of other sugar molecule , the ${\text{OH}}$ molecule attached to the anomeric carbon is removed along with the ${\text{H}}$ of the alcohol. That is both ${\text{OH}}$ and ${\text{H}}$ are removed from the original molecule during the reaction and form glycosidic bonds.

We already know that Carbohydrates are mainly classified into monosaccharides, oligosaccharides and polysaccharides. Where monosaccharides are simple carbohydrates. Both glucose and fructose belong to this group.


For both glucose and fructose there is no glycosidic linkage as it is clear from the structures.
Oligosaccharides are carbohydrates chains containing two to ten sugar units. In which the monosaccharides are joined together by glycosidic linkage. Sucrose comes under this group. And its structure is given by

Sucrose is composed of two monosaccharides glucose and fructose , where the two monosaccharides are joined by the glycosidic linkage.
Next is starch, which comes under the polysaccharides group. Where polysaccharides are the long chain of monosaccharides that are linked by glycosidic linkage.

So it is clear that in sucrose and starch glycosidic linkage is present.
so, the correct answer is option A,B..
Note: glycosidic bonds come in different forms and they can be named according to the number of carbon atoms of the sugar molecule which are involved in the linkage. For example 1,4-glycosidic bonds , in which number one carbon atom of one sugar molecule and number fourth carbon atom of other sugar molecule participate in bond formation. There is also a 1,6-glycosidic bond , where again the number one carbon atom of sugar molecule and sixth carbon atom of other sugar molecule is involved.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




