
What is ‘Glycerose’? How is it prepared from glycerol?
Answer
578.4k+ views
Hint: The Glycerose is the mixture of two compounds forming from the oxidation of glycerol.
Complete step by step:
Let us know about the glycerol and glycerose first.
Glycerol- It is also called glycerine. It is a non-toxic compound which is sweet in taste. It is usually in liquid form which is odourless, viscous and colourless. It has antiviral and anti-microbial properties.
Due to its remarkable properties, it is primarily used in food and pharmaceutical industries.
Glycerose- The aldose and ketose (here, glyceraldehyde and dihydroxyacetone respectively) form a mixture equilibrium, which is called glycerose. This can be obtained from glycerol.
Mild oxidising agent like bromine water or Fenton’s reagent $\left[ FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}} \right]$ or sodium hypobromite oxidises the glycerol which results the mixture called glycerose.
The reaction is given as,
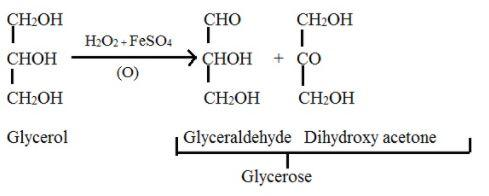
Additional information:
Fenton’s reagent- It is a solution of hydrogen peroxide with ferrous ions. It is mostly used as the catalyst for oxidising contaminants in the water. Fenton’s reagent is used in organic synthesis of phenol from benzene as,
\[{{C}_{6}}{{H}_{6}}+FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\to {{C}_{6}}{{H}_{5}}OH\]
Note: There are three reagents to produce glycerose from glycerol. Here, only one is shown, but the reactants are products are the same for all the reactions.
Complete step by step:
Let us know about the glycerol and glycerose first.
Glycerol- It is also called glycerine. It is a non-toxic compound which is sweet in taste. It is usually in liquid form which is odourless, viscous and colourless. It has antiviral and anti-microbial properties.
Due to its remarkable properties, it is primarily used in food and pharmaceutical industries.
Glycerose- The aldose and ketose (here, glyceraldehyde and dihydroxyacetone respectively) form a mixture equilibrium, which is called glycerose. This can be obtained from glycerol.
Mild oxidising agent like bromine water or Fenton’s reagent $\left[ FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}} \right]$ or sodium hypobromite oxidises the glycerol which results the mixture called glycerose.
The reaction is given as,

Additional information:
Fenton’s reagent- It is a solution of hydrogen peroxide with ferrous ions. It is mostly used as the catalyst for oxidising contaminants in the water. Fenton’s reagent is used in organic synthesis of phenol from benzene as,
\[{{C}_{6}}{{H}_{6}}+FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\to {{C}_{6}}{{H}_{5}}OH\]
Note: There are three reagents to produce glycerose from glycerol. Here, only one is shown, but the reactants are products are the same for all the reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




