
Glycerol reacts with potassium bisulphate to produce ____________
A. Allyl iodide
B. Allyl sulphate
C. Acrylaldehydeṣ
D. Glycerol trisulphate
Answer
560.7k+ views
Hint: The mole is considered as the basic unit for measuring or expressing the amount of the substance. It is defined as the amount of substance which is equal to the substance present in 12g of carbon 12 atom. We can calculate the moles of the substance by dividing the mass of the substance by the molar mass.
Complete step by step answer:
Let us first see what is Glycerol and potassium bisulphate.
Glycerol (called glycerine) is a simple polyol compound. It is a colourless, odourless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in those lipids known as glycerides.
Given below is the structure of Glycerol
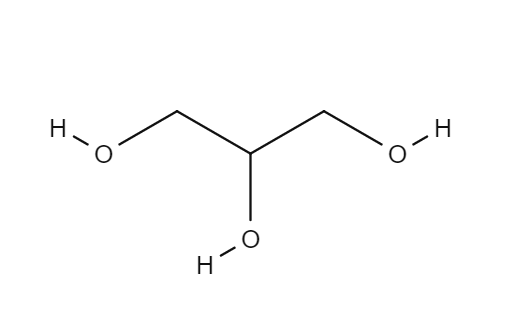
Glycerol is most commonly used for constipation, improving hydration and performance in athletes, and for certain skin conditions. It is also used for meningitis, stroke, obesity, ear infections, and other conditions, but there is no good scientific evidence to support these uses.
Potassium bisulphate is an inorganic compound with the chemical formula $KHSO_4$ and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid, used as a preservative, as a flux and also in the manufacture of fertilizers. Glycerol on heating potassium Bisulphate, undergoes dehydration, that is removal of two water molecules from the glycerol molecule. Hence, resulting in formation of unsaturated aldehyde i.e. acrylic aldehyde.
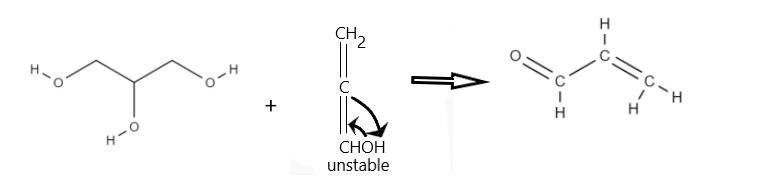
Hence the product formed is acrylic aldehyde, also called acrolein. Therefore, option C is the correct answer.
So, the correct answer is Option C.
Additional information:
Acrolein is mostly used to make acrylic acid. It is also used to control plant and algae growth in irrigation canals. Acrolein kills or controls microorganisms and bacteria in oil wells, liquid hydrocarbon fuels, cooling-water towers and water treatment ponds. In papermaking, acrolein is used to control slime.
Note: The glycerol is used in the food industry as a humectant. It is used as sweetening and a solvent for several beverages. It is also used in the pharmaceutical industry in treatment of wounds and burns. The glycerol dehydration is mainly carried out in gaseous phase in the presence of an acid catalyst such as protonated or metal-promoted zeolites, mixed metallic oxides, functionalized oxides, or supported heteropolyacids, at atmospheric pressure and reaction temperatures between 453 and 773 K.
Complete step by step answer:
Let us first see what is Glycerol and potassium bisulphate.
Glycerol (called glycerine) is a simple polyol compound. It is a colourless, odourless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in those lipids known as glycerides.
Given below is the structure of Glycerol

Glycerol is most commonly used for constipation, improving hydration and performance in athletes, and for certain skin conditions. It is also used for meningitis, stroke, obesity, ear infections, and other conditions, but there is no good scientific evidence to support these uses.
Potassium bisulphate is an inorganic compound with the chemical formula $KHSO_4$ and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid, used as a preservative, as a flux and also in the manufacture of fertilizers. Glycerol on heating potassium Bisulphate, undergoes dehydration, that is removal of two water molecules from the glycerol molecule. Hence, resulting in formation of unsaturated aldehyde i.e. acrylic aldehyde.

Hence the product formed is acrylic aldehyde, also called acrolein. Therefore, option C is the correct answer.
So, the correct answer is Option C.
Additional information:
Acrolein is mostly used to make acrylic acid. It is also used to control plant and algae growth in irrigation canals. Acrolein kills or controls microorganisms and bacteria in oil wells, liquid hydrocarbon fuels, cooling-water towers and water treatment ponds. In papermaking, acrolein is used to control slime.
Note: The glycerol is used in the food industry as a humectant. It is used as sweetening and a solvent for several beverages. It is also used in the pharmaceutical industry in treatment of wounds and burns. The glycerol dehydration is mainly carried out in gaseous phase in the presence of an acid catalyst such as protonated or metal-promoted zeolites, mixed metallic oxides, functionalized oxides, or supported heteropolyacids, at atmospheric pressure and reaction temperatures between 453 and 773 K.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




