
When glycerol is treated with excess hydrogen iodide, it produces?
A. Allyl iodide.
B. Propane.
C. Glycerol triiodide.
D. 2-Iodopropane.
Answer
587.1k+ views
Hint: We know, Glycerol is a colorless, odorless liquid. It is also called Glycerin. The IUPAC of glycerol is$propane - 1,2,3, - triol$. Glycerol has a main application in treating wounds and burns as it has antiviral and antifungal properties. Synthetic glycerin obtained from the chemical process of petroleum, chlorine and propylene and natural glycerin produced at animal or vegetable fats by the hydrolysis process.
Complete step by step answer: We must remember that the glycerol has many alcohols so is also called a polyol. It is soluble in water and it has a tendency to attract the water molecules that are hygroscopic.
If we treat the glycerol with an excess of hydrogen iodide it forms $1,2,3 - tri-iodopropane$ t the first step and it is unstable. It loses a molecule of iodine and forms Allyl iodide. Then hydrogen iodide is added to form Allyl iodide and to form an unstable molecule and it loses a molecule of iodine to form propene. The formed propene undergoes an additional reaction with HI molecule to form $2 - iodopropane$.
We can write chemical equation for the reaction of glycerol with hydrogen iodide as,
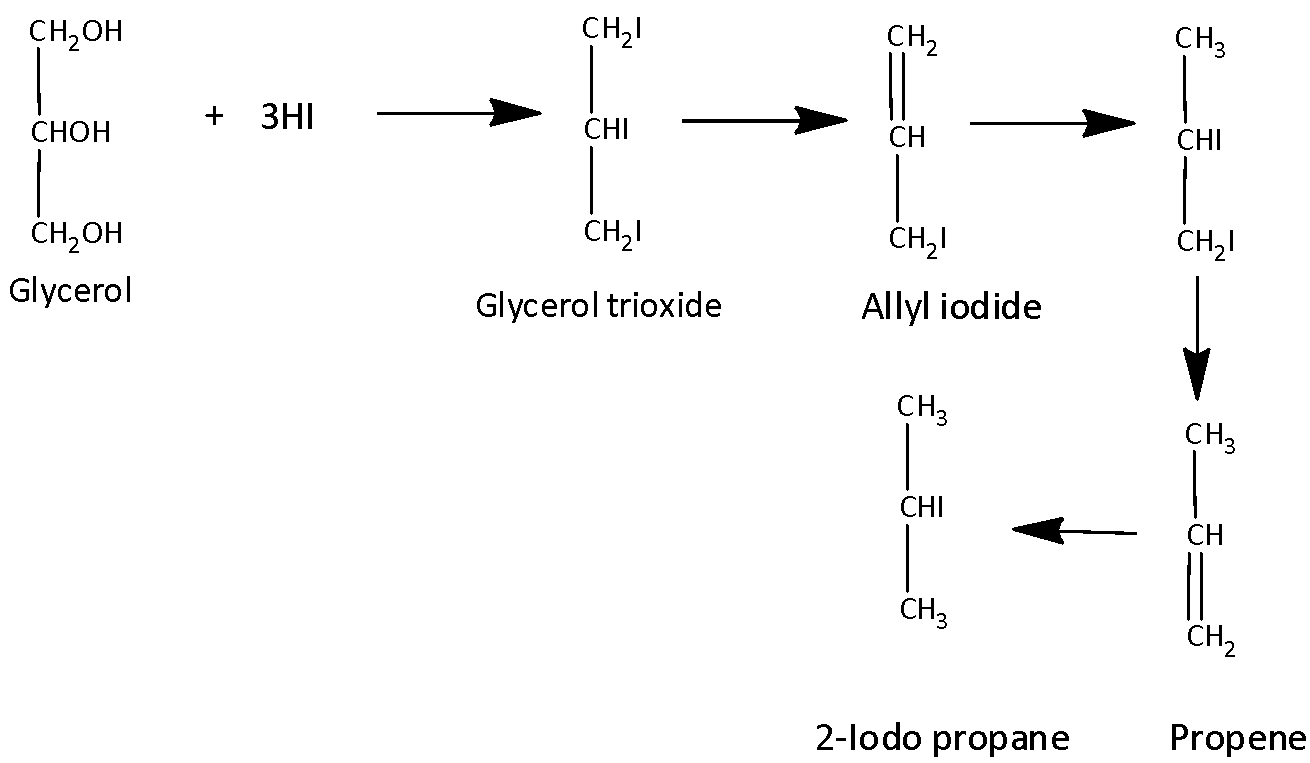
.
So, the correct answer is “Option D”.
Note: Now we can discuss about the uses of glycerin in various field:
-The major application of glycerin is in the food and beverage industry, it is employed as a sweetener, and also as a solvent.
-Glycerin is also used as a preservative for storing food.
-It is also used in liqueurs as a thickening agent
-As a lubricant and humectants, glycerin is used in the medical and pharmaceutical industries
-In film industries, Glycerin is used to avoid the quick drying of wet areas.
Complete step by step answer: We must remember that the glycerol has many alcohols so is also called a polyol. It is soluble in water and it has a tendency to attract the water molecules that are hygroscopic.
If we treat the glycerol with an excess of hydrogen iodide it forms $1,2,3 - tri-iodopropane$ t the first step and it is unstable. It loses a molecule of iodine and forms Allyl iodide. Then hydrogen iodide is added to form Allyl iodide and to form an unstable molecule and it loses a molecule of iodine to form propene. The formed propene undergoes an additional reaction with HI molecule to form $2 - iodopropane$.
We can write chemical equation for the reaction of glycerol with hydrogen iodide as,

.
So, the correct answer is “Option D”.
Note: Now we can discuss about the uses of glycerin in various field:
-The major application of glycerin is in the food and beverage industry, it is employed as a sweetener, and also as a solvent.
-Glycerin is also used as a preservative for storing food.
-It is also used in liqueurs as a thickening agent
-As a lubricant and humectants, glycerin is used in the medical and pharmaceutical industries
-In film industries, Glycerin is used to avoid the quick drying of wet areas.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




