
Glucose contains:
A. One $ - {{CHO}}$ group
B. Five $ - {{OH}}$ group
C. One primary alcohol group
D. All are correct.
Answer
558.6k+ views
Hint: Glucose is an example of hydrocarbon. It contains six carbon atoms. The chemical formula of glucose is ${{{C}}_6}{{{H}}_{12}}{{{O}}_6}$. It comes under the group of carbohydrates. Glucose has a hydrogen atom in it. But it is not considered as an acid.
Complete step by step answer:
Glucose is an example of simple sugar. It is an organic compound. They are of two forms-D and L form. These are the stereoisomers of glucose. Apart from six carbon atoms, glucose has twelve hydrogen atoms and six oxygen atoms. Glucose is a type of monosaccharide which cannot be hydrolyzed further.
The chemical structure of glucose is given below:
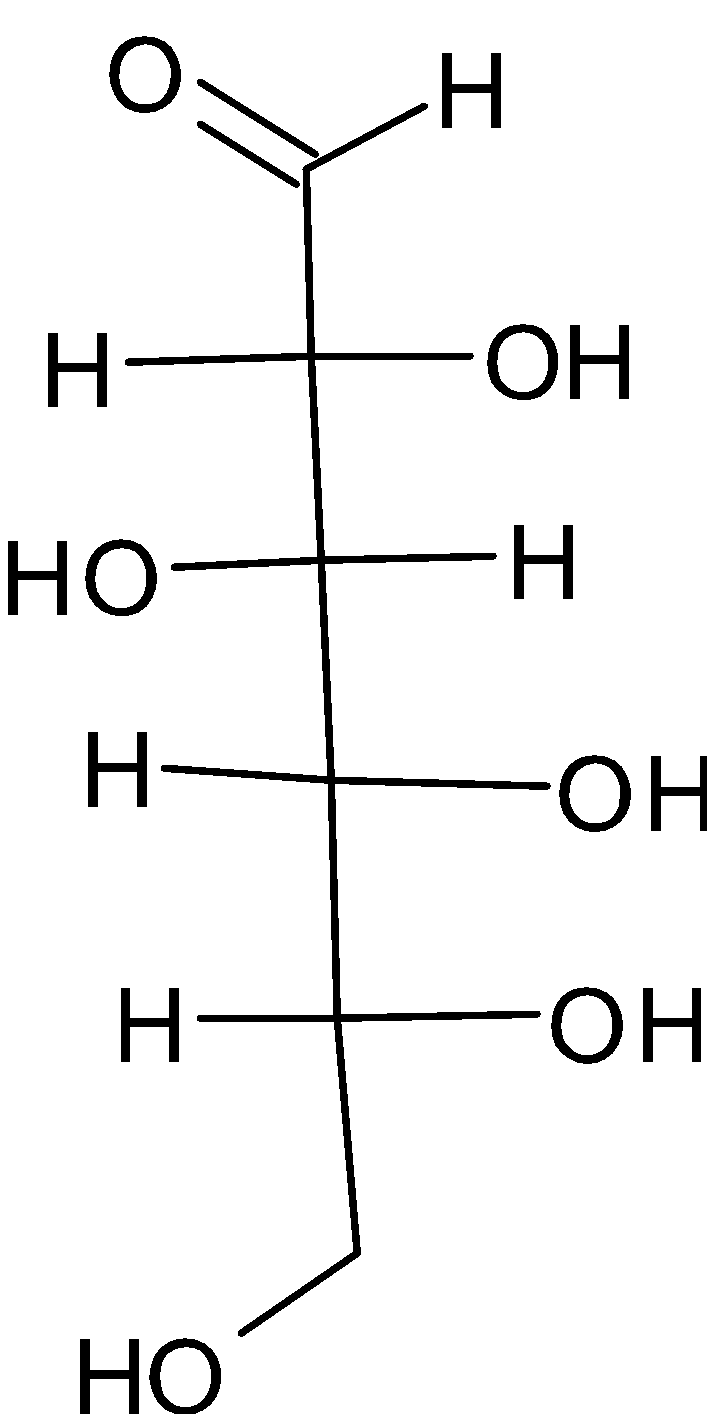
From the figure, it is understood that glucose has five hydroxyl groups which are attached to the carbon atom. An aldehyde group is attached to the sixth carbon atom. This compound is termed as aldohexose. Aldo is used because there is an aldehyde group present in glucose. While hexose is used because there is a six carbon chain. It is considered as the fundamental unit of carbohydrates.
From the structure, we can determine whether these given groups are there or not. It has one aldehyde group which is represented by $ - {{CHO}}$. It also has five hydroxyl groups which are attached to the second, third, fourth, fifth and sixth carbon atom. It has one primary alcoholic group which is at the sixth carbon atom.
Thus all the statements are correct.
So, the correct option is D.
Note: We may confuse aldohexose and ketohexose because both of them have a carbonyl group attached to the carbon chain. But both of them are different. If there is a monosaccharide having a ketone group, then it is termed as ketohexose.
Complete step by step answer:
Glucose is an example of simple sugar. It is an organic compound. They are of two forms-D and L form. These are the stereoisomers of glucose. Apart from six carbon atoms, glucose has twelve hydrogen atoms and six oxygen atoms. Glucose is a type of monosaccharide which cannot be hydrolyzed further.
The chemical structure of glucose is given below:

From the figure, it is understood that glucose has five hydroxyl groups which are attached to the carbon atom. An aldehyde group is attached to the sixth carbon atom. This compound is termed as aldohexose. Aldo is used because there is an aldehyde group present in glucose. While hexose is used because there is a six carbon chain. It is considered as the fundamental unit of carbohydrates.
From the structure, we can determine whether these given groups are there or not. It has one aldehyde group which is represented by $ - {{CHO}}$. It also has five hydroxyl groups which are attached to the second, third, fourth, fifth and sixth carbon atom. It has one primary alcoholic group which is at the sixth carbon atom.
Thus all the statements are correct.
So, the correct option is D.
Note: We may confuse aldohexose and ketohexose because both of them have a carbonyl group attached to the carbon chain. But both of them are different. If there is a monosaccharide having a ketone group, then it is termed as ketohexose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




