
Given the structural formula for the following: Ethanal.
Answer
590.1k+ views
Hint: Firstly, we need to know that Ethanal is the IUPAC name of Acetaldehyde. Acetaldehyde is an organic chemical compound which is one of the most common aldehydes widely available in nature.
Complete step by step answer:
First, we need to remember that ethanol i.e. acetaldehyde which is chemically written as $C{H_3}CHO$ is used as the initial substance in the synthesis of acetic acid, ethyl acetate, n-butyl alcohol, and some other chemical compounds. As we know that, ethanol is manufactured by the oxidation of ethyl alcohol. Also, it is manufactured by the catalytic hydration of acetylene.
Now, we need to understand how aldehydes and ketones are formed using catalytic dehydrogenation of alcohols. As soon as the vapors of any primary or secondary alcohol is passed or brought in contact to heated Copper at 573 K then the process of dehydrogenation occurs. After dehydrogenation, an aldehyde and ketone can be formed.
As ethanal belongs to the functional group of $ - CHO$ group i.e., aldehydes so their parent hydrocarbon is ethane. So, the chemical formula of ethane is ${C_2}{H_6}.$ Now, we need to add the $ - CHO$ group to draw the structural formula of ethanal.
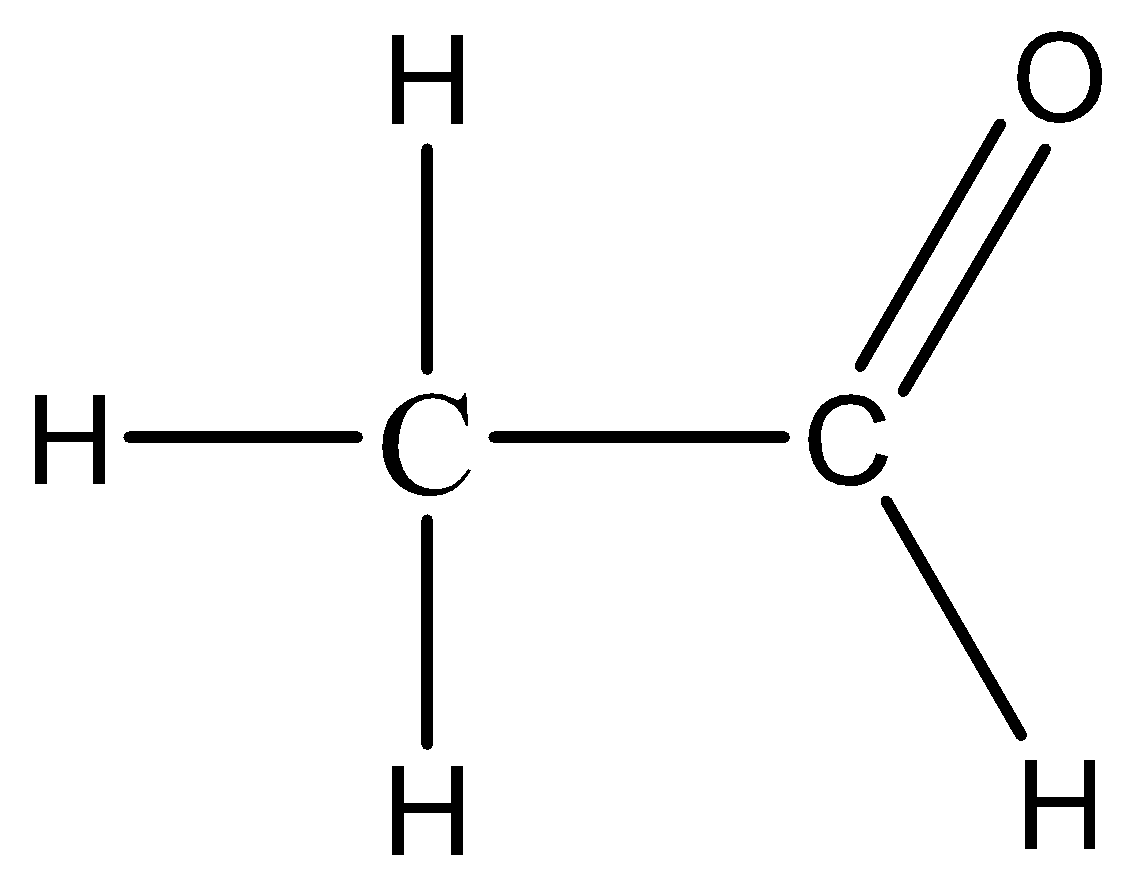
Thus, the structural formula of ethanal is ${C_2}{H_4}O.$
Note:
We must know that the Ethanal is a colorless and flammable liquid. We can use it in silver mirrors and in breathalyzer testing. Also, we need to remember that ethane is an alkane which is a saturated hydrocarbon whereas ethane and ethyne are alkene and alkyne respectively so they are unsaturated hydrocarbons. Ethanal comes from the class of organic compounds which are popularly known as short-chain aldehydes.
Complete step by step answer:
First, we need to remember that ethanol i.e. acetaldehyde which is chemically written as $C{H_3}CHO$ is used as the initial substance in the synthesis of acetic acid, ethyl acetate, n-butyl alcohol, and some other chemical compounds. As we know that, ethanol is manufactured by the oxidation of ethyl alcohol. Also, it is manufactured by the catalytic hydration of acetylene.
Now, we need to understand how aldehydes and ketones are formed using catalytic dehydrogenation of alcohols. As soon as the vapors of any primary or secondary alcohol is passed or brought in contact to heated Copper at 573 K then the process of dehydrogenation occurs. After dehydrogenation, an aldehyde and ketone can be formed.
As ethanal belongs to the functional group of $ - CHO$ group i.e., aldehydes so their parent hydrocarbon is ethane. So, the chemical formula of ethane is ${C_2}{H_6}.$ Now, we need to add the $ - CHO$ group to draw the structural formula of ethanal.

Thus, the structural formula of ethanal is ${C_2}{H_4}O.$
Note:
We must know that the Ethanal is a colorless and flammable liquid. We can use it in silver mirrors and in breathalyzer testing. Also, we need to remember that ethane is an alkane which is a saturated hydrocarbon whereas ethane and ethyne are alkene and alkyne respectively so they are unsaturated hydrocarbons. Ethanal comes from the class of organic compounds which are popularly known as short-chain aldehydes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




