
Given:
\[{{O}_{2}}(g)+4{{H}^{+}}(aq)+4{{e}^{-}}\to 2{{H}_{2}}O(l)\]
\[{{E}^{\oplus }}=1.23\text{ V}\]
\[{{\text{S}}_{2}}{{O}_{8}}^{2-}(aq)+2{{e}^{-}}\to 2S{{O}_{4}}^{2-}(aq)\]
\[{{E}^{-}}=2.01\text{ V}\]
Assertion: Peroxodisulphate salts (e.g.\[\text{N}{{\text{a}}_{2}}{{S}_{2}}{{O}_{8}}\] ) are strong oxidizing agents used as bleaching agents for fats, oils and fabrics.
Reason: Oxygen gas can oxidize sulphate ion to peroxodisulphate ion (\[{{\text{S}}_{2}}{{O}_{8}}^{2-}\] ) in acidic solution, with the \[{{O}_{2}}(g)\] being reduced to water.
(A)- Both assertion and reason are true and reason is the correct explanation of assertion
(B)- Both assertion and reason a true but reason is not the correct explanation of assertion
(C)- Assertion is true but reason is false
(D)- Assertion is false but reason is true
Answer
594.6k+ views
Hint:
Higher the value of the reduction potential of the corresponding electrode (cathode or anode), more is the tendency of the electrode to participate in the reduction reaction. Reduction is the reaction in which there is decrease in the oxidation state or the gain of electrons.
Complete step by step solution:
- Peroxodisulphate salts (e.g.\[\text{N}{{\text{a}}_{2}}{{S}_{2}}{{O}_{8}}\] ) are strong oxidizing agents used as bleaching agents for fats, oils and fabrics.
- In Peroxodisulfate salts, sulphur is in +7 oxidation state and hence it can be reduced to lesser oxidation states. That means it can act as a strong oxidising agent.
Thus, the assertion is a true sentence.
- It is proved that in a galvanic cell for a redox reaction to be spontaneous or feasible, \[{{E}^{\circ }}_{cell}\] must be greater than zero.
- \[{{E}^{\circ }}_{cell}\] is the total resultant reduction potential which is the difference of the reduction potential of the cathode and the reduction potential of the anode . It is calculated as follows:
\[{{E}^{\circ }}_{cell}={{E}^{\oplus }}-{{E}^{-}}\] .....(1)
- Since, the value of the reduction potential of the second redox reaction is more than that of the first reaction, so the second one is the reduction reaction. The first one is the oxidation reaction.
- Let’s write anode and cathode reactions for the oxidation of sulphate ions by oxygen.
Reaction at Cathode: \[{{O}_{2}}(g)+4{{H}^{+}}(aq)+4{{e}^{-}}\to 2{{H}_{2}}O(l)\]
Reaction at Anode: \[2S{{O}_{4}}^{2-}(aq)\to {{\text{S}}_{2}}{{O}_{8}}^{2-}(aq)+2{{e}^{-}}\]
Now, to write the total reaction, we will need to multiply anodic reaction by 2 because it involves change of 2 electrons while cathodic reaction involves change of 4 electrons.
Total reaction : \[{{O}_{2}}(g)+4{{H}^{+}}(aq)+4S{{O}_{4}}^{2-}(aq)\to 2{{H}_{2}}O(l)+2{{\text{S}}_{2}}{{O}_{8}}^{2-}(aq)\]
Now, for this reaction, we can use equation (1) to predict ${{E}^{\circ }}_{cell}$ .
So, \[{{E}^{\circ }}_{cell}={{E}^{\oplus }}-{{E}^{-}}\]
Now, we know that for, cathode reaction, standard reduction potential is given as 1.23V (${{E}^{\oplus }}$) and for anode reaction, standard reduction potential is given as 2.01V (${{E}^{-}}$) .
So, for this cell, we can write that ${{E}^{\circ }}_{cell}=1.23-2.01$
So, \[{{E}^{\circ }}_{cell}=-0.78V\]
Thus, the resultant potential of this cell will be in less than zero and thus we can say that this reaction will not occur without giving any external energy.
- So, we can say that the reason is not a correct sentence.
Thus, the assertion is true but the reason is not correct.
So, option C is the correct answer.
Note:
Remember that in equation (1), which is given in the complete solution part, we should put the values of standard reduction potential of the half cell there in both cathode and anode. In peroxodisulphate ion (\[{{\text{S}}_{2}}{{O}_{8}}^{2-}\] ), the oxidation state of oxygen in the O-O linkage is -1 and not -2. So there are six oxygen with -2 oxidation state and the rest two have -1 oxidation state.
Structure of \[{{\text{S}}_{2}}{{O}_{8}}^{2-}\] :
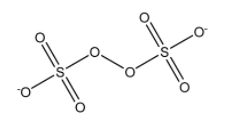
In the sulphate ion, the oxidation state is the oxygen is -2
Higher the value of the reduction potential of the corresponding electrode (cathode or anode), more is the tendency of the electrode to participate in the reduction reaction. Reduction is the reaction in which there is decrease in the oxidation state or the gain of electrons.
Complete step by step solution:
- Peroxodisulphate salts (e.g.\[\text{N}{{\text{a}}_{2}}{{S}_{2}}{{O}_{8}}\] ) are strong oxidizing agents used as bleaching agents for fats, oils and fabrics.
- In Peroxodisulfate salts, sulphur is in +7 oxidation state and hence it can be reduced to lesser oxidation states. That means it can act as a strong oxidising agent.
Thus, the assertion is a true sentence.
- It is proved that in a galvanic cell for a redox reaction to be spontaneous or feasible, \[{{E}^{\circ }}_{cell}\] must be greater than zero.
- \[{{E}^{\circ }}_{cell}\] is the total resultant reduction potential which is the difference of the reduction potential of the cathode and the reduction potential of the anode . It is calculated as follows:
\[{{E}^{\circ }}_{cell}={{E}^{\oplus }}-{{E}^{-}}\] .....(1)
- Since, the value of the reduction potential of the second redox reaction is more than that of the first reaction, so the second one is the reduction reaction. The first one is the oxidation reaction.
- Let’s write anode and cathode reactions for the oxidation of sulphate ions by oxygen.
Reaction at Cathode: \[{{O}_{2}}(g)+4{{H}^{+}}(aq)+4{{e}^{-}}\to 2{{H}_{2}}O(l)\]
Reaction at Anode: \[2S{{O}_{4}}^{2-}(aq)\to {{\text{S}}_{2}}{{O}_{8}}^{2-}(aq)+2{{e}^{-}}\]
Now, to write the total reaction, we will need to multiply anodic reaction by 2 because it involves change of 2 electrons while cathodic reaction involves change of 4 electrons.
Total reaction : \[{{O}_{2}}(g)+4{{H}^{+}}(aq)+4S{{O}_{4}}^{2-}(aq)\to 2{{H}_{2}}O(l)+2{{\text{S}}_{2}}{{O}_{8}}^{2-}(aq)\]
Now, for this reaction, we can use equation (1) to predict ${{E}^{\circ }}_{cell}$ .
So, \[{{E}^{\circ }}_{cell}={{E}^{\oplus }}-{{E}^{-}}\]
Now, we know that for, cathode reaction, standard reduction potential is given as 1.23V (${{E}^{\oplus }}$) and for anode reaction, standard reduction potential is given as 2.01V (${{E}^{-}}$) .
So, for this cell, we can write that ${{E}^{\circ }}_{cell}=1.23-2.01$
So, \[{{E}^{\circ }}_{cell}=-0.78V\]
Thus, the resultant potential of this cell will be in less than zero and thus we can say that this reaction will not occur without giving any external energy.
- So, we can say that the reason is not a correct sentence.
Thus, the assertion is true but the reason is not correct.
So, option C is the correct answer.
Note:
Remember that in equation (1), which is given in the complete solution part, we should put the values of standard reduction potential of the half cell there in both cathode and anode. In peroxodisulphate ion (\[{{\text{S}}_{2}}{{O}_{8}}^{2-}\] ), the oxidation state of oxygen in the O-O linkage is -1 and not -2. So there are six oxygen with -2 oxidation state and the rest two have -1 oxidation state.
Structure of \[{{\text{S}}_{2}}{{O}_{8}}^{2-}\] :

In the sulphate ion, the oxidation state is the oxygen is -2
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




