
Give the structure and IUPAC name of metamers of 2-methoxypropane.
Answer
562.8k+ views
Hint:To solve this we must know the metamers. The compounds that have the same molecular formula but different number of carbon atoms or alkyl groups on either side of the functional group are called metamers. This phenomenon is known as metamerism.
Complete step-by-step answer:We are given 2-methoxypropane.
In 2-methoxypropane, propane suggests that the parent alkane is propane. Thus, the longest continuous carbon chain has three carbon atoms. And 2-methoxy suggests that methoxy $\left( {{\text{O}} - {\text{C}}{{\text{H}}_3}} \right)$ group is attached to the carbon number 2 of the chain.
Thus, the structure of 2-methoxypropane is as follows:
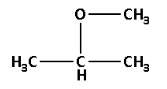
2-methoxy propane has ester $\left( { - {\text{O}} - } \right)$ functional group. The molecular formula for 2-methoxypropane is ${{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}$.
We know that the compounds that have same molecular formula but different number of carbon atoms or alkyl groups on either side of the functional group are called metamers. This phenomenon is known as metamerism.
The compound having molecular formula ${{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}$ but having different number of carbon atoms or alkyl groups on either side of the functional group $\left( { - {\text{O}} - } \right)$ is the metamer of 2-methoxypropane.
Thus, the structure of metamer of 2-methoxypropane is as follows:
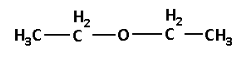
The metamer of 2-methoxypropane has two ethyl groups on the both sides of the ester $\left( { - {\text{O}} - } \right)$ functional group.
Thus, the IUPAC name of the metamer of 2-methoxypropane is diethyl ether. Diethyl ether is also known as ethoxy ethane.
The other metamer of 2-methoxypropane is 1-methoxypropane. The structure of 1-methoxypropane is as follows:
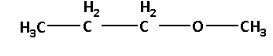
Note: Do not confuse between tautomers and metamers. Tautomers have the same molecular formula and are readily interconvertible. Whereas metamers have the same molecular formula but different numbers of carbon atoms or alkyl groups on either side of the functional group.
Complete step-by-step answer:We are given 2-methoxypropane.
In 2-methoxypropane, propane suggests that the parent alkane is propane. Thus, the longest continuous carbon chain has three carbon atoms. And 2-methoxy suggests that methoxy $\left( {{\text{O}} - {\text{C}}{{\text{H}}_3}} \right)$ group is attached to the carbon number 2 of the chain.
Thus, the structure of 2-methoxypropane is as follows:

2-methoxy propane has ester $\left( { - {\text{O}} - } \right)$ functional group. The molecular formula for 2-methoxypropane is ${{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}$.
We know that the compounds that have same molecular formula but different number of carbon atoms or alkyl groups on either side of the functional group are called metamers. This phenomenon is known as metamerism.
The compound having molecular formula ${{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}$ but having different number of carbon atoms or alkyl groups on either side of the functional group $\left( { - {\text{O}} - } \right)$ is the metamer of 2-methoxypropane.
Thus, the structure of metamer of 2-methoxypropane is as follows:
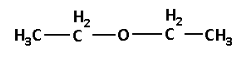
The metamer of 2-methoxypropane has two ethyl groups on the both sides of the ester $\left( { - {\text{O}} - } \right)$ functional group.
Thus, the IUPAC name of the metamer of 2-methoxypropane is diethyl ether. Diethyl ether is also known as ethoxy ethane.
The other metamer of 2-methoxypropane is 1-methoxypropane. The structure of 1-methoxypropane is as follows:
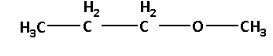
Note: Do not confuse between tautomers and metamers. Tautomers have the same molecular formula and are readily interconvertible. Whereas metamers have the same molecular formula but different numbers of carbon atoms or alkyl groups on either side of the functional group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




