
Give the major product of the following reaction:
3, 3- dimethyl-1-butene $ + HCl \to Major$
A. 2-chloro-3,3-dimethyl butane
B. 2-chloro-2,3-dimethyl butane
C. 1,2- dichloro-3,3-dimethylbutane
D. 1-chloro-3,3-dimethylbutane
Answer
565.2k+ views
Hint: Organic compounds are the compounds which in solid, liquid, and gaseous state contain carbon in its molecule. A large number of organic compounds are there and a systematic classification is required. Organic compounds are classified as open-chain (acyclic), closed chain (cyclic). Open chain is classified into two types: straight-chain and branched-chain. The closed chain is classified into two types: Homocyclic compounds and Heterocyclic compounds. Homocyclic compounds are classified into two types: Alicyclic compounds and Aromatic compounds.
Complete step by step answer:
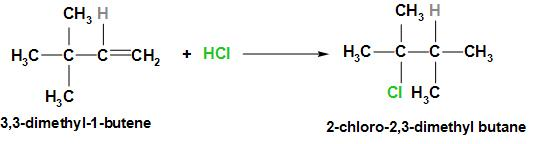
When 3, 3- dimethyl-1-butene reacts with HCl it gives 2-chloro-2, 3-dimethyl butane as the major product. The reaction along with the mechanism is written below:
Mechanism:
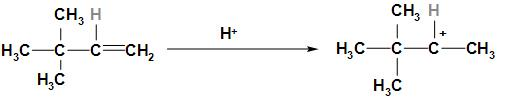
Step 1: In this step, the double bond in 3, 3- dimethyl-1-butene breaks and it forms a secondary carbocation, and the ${H^ + }$ ion of HCl gets added to the negative carbon.
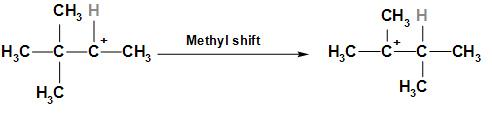
Step 2: In this step one methyl group gets shifted to secondary carbocation to form tertiary carbocation which is the most stable carbocation.
Step 3: The $C{l^ - }$ ion will be added to the tertiary carbocation to form the major product 2-chloro-2, 3-dimethyl butane.
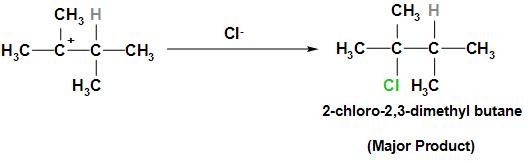
Therefore the major product is 2-chloro-2, 3-dimethyl butane.
Hence, the correct option is B.
Note: When a group X, having electronegativity higher than carbon is attached to it, $\left( {C - X} \right)$ bond breaks heterolytically. The shared pair of electrons is given to the atom other than the carbon. Thus, the carbon atom acquires a positive charge. It has only six electrons in its valence shell. Such a type of ion of carbon is known as carbocation or carbonium ion. The positively charged ion of the carbocation is $s{p^2}$ hybridized.
Complete step by step answer:
When 3, 3- dimethyl-1-butene reacts with HCl it gives 2-chloro-2, 3-dimethyl butane as the major product. The reaction along with the mechanism is written below:

Mechanism:
Step 1: In this step, the double bond in 3, 3- dimethyl-1-butene breaks and it forms a secondary carbocation, and the ${H^ + }$ ion of HCl gets added to the negative carbon.

Step 2: In this step one methyl group gets shifted to secondary carbocation to form tertiary carbocation which is the most stable carbocation.

Step 3: The $C{l^ - }$ ion will be added to the tertiary carbocation to form the major product 2-chloro-2, 3-dimethyl butane.

Therefore the major product is 2-chloro-2, 3-dimethyl butane.
Hence, the correct option is B.
Note: When a group X, having electronegativity higher than carbon is attached to it, $\left( {C - X} \right)$ bond breaks heterolytically. The shared pair of electrons is given to the atom other than the carbon. Thus, the carbon atom acquires a positive charge. It has only six electrons in its valence shell. Such a type of ion of carbon is known as carbocation or carbonium ion. The positively charged ion of the carbocation is $s{p^2}$ hybridized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




