
Give the diazotization reaction of aniline. Also, give the chemical reaction involved in the preparation of red azo dye and light yellow azo dye.
Answer
594.9k+ views
Hint: The conversion of a primary amine into its diazonium salt is called diazotization. The temperature required for this reaction is in the range of 273K to 278K.
Complete step by step answer: Aromatic diazonium salt is generally prepared by adding a cold aqueous solution of sodium nitrite to the solution or suspension of a primary aromatic amine in acid at 273K to 278K. The general reaction is written as:
$ArN{H_2} + NaN{O_2} + 2HX\xrightarrow{{273K - 278K}}ArN_2^ + {X^ - } + NaX + 2{H_2}O$
Where Ar is an aryl group and X is halogen.
In the case of aniline diazotization reaction is written as:

Arenediazonium salts react with a highly reactive aromatic compound such as phenol and amines to form brightly colored azo compounds. This reaction is known as a coupling reaction.
The chemical reaction involved in the preparation of red azo dye:
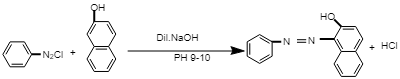
In this reaction, diazonium salt reacts with β-Naphthol in the presence of dil. NaOH at PH 9-10, i.e. in alkali medium to give β-Naphthol aniline dye, i.e. red azo dye.
The chemical reaction involved in the formation of yellow dye:
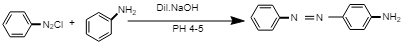
In this reaction, diazonium salt reacts with aniline in the presence of dil. NaOH at PH 4-5, i.e. in acidic medium to give p-Aminoazobenzene, i.e. yellow dye.
Note: The well-known indicator methyl orange used in acid-base titration is also prepared by a coupling reaction. So we can say diazonium salts are very good intermediates.
Complete step by step answer: Aromatic diazonium salt is generally prepared by adding a cold aqueous solution of sodium nitrite to the solution or suspension of a primary aromatic amine in acid at 273K to 278K. The general reaction is written as:
$ArN{H_2} + NaN{O_2} + 2HX\xrightarrow{{273K - 278K}}ArN_2^ + {X^ - } + NaX + 2{H_2}O$
Where Ar is an aryl group and X is halogen.
In the case of aniline diazotization reaction is written as:

Arenediazonium salts react with a highly reactive aromatic compound such as phenol and amines to form brightly colored azo compounds. This reaction is known as a coupling reaction.
The chemical reaction involved in the preparation of red azo dye:

In this reaction, diazonium salt reacts with β-Naphthol in the presence of dil. NaOH at PH 9-10, i.e. in alkali medium to give β-Naphthol aniline dye, i.e. red azo dye.
The chemical reaction involved in the formation of yellow dye:

In this reaction, diazonium salt reacts with aniline in the presence of dil. NaOH at PH 4-5, i.e. in acidic medium to give p-Aminoazobenzene, i.e. yellow dye.
Note: The well-known indicator methyl orange used in acid-base titration is also prepared by a coupling reaction. So we can say diazonium salts are very good intermediates.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




