
Give reasons for why:
(i) Alkyl halides are insoluble in water even though they possess a polar $C-X$bond.
(ii) Grignard Reagent should be prepared under anhydrous conditions.
(iii) Vinyl chloride is less reactive than ethyl chloride towards nucleophilic substitutions.
Answer
596.1k+ views
Hint: Dissolving alkyl Halides in water results in new bonds of attraction between alkyl Halides and water molecules. This process releases little energy implying weak bond strength.
Water is a proton donating solvent which hinders the existence of Grignard’s reagent.
Vinyl chloride’s structure contains a double bond whereas no such bond exists in ethyl chloride.
Complete step by step solution:
Let us go through the answer this question each part at a time:
(i) Alkyl halides are organic compounds. Water is a polar solvent having a high dielectric constant, which means it can separate charges from each other. This makes a compound dissolve in water. Compounds which are polar in nature are therefore soluble in water. Alkyl halides are polar only due to the halogen substituent. The alkyl part is hydrophobic in nature which repels water molecules. The bigger this hydrophobic part is the more insoluble that alkyl halide becomes. Hence, smaller alkyl halides are fairly soluble in water.
This phenomenon can be explained in yet another way. For a substance to dissolve in water, it has to form interactions with water molecules that are more stable than the interactions it already has. Only then there will be a net release of energy and the compound will dissolve. This will never happen in case of alkyl halides as:
- The covalent bonds are very strong and therefore not easy to break.
- As the hydrocarbon part of the alkyl halide is hydrophobic in nature, it will always repel water, making the new interaction weaker.
(ii) Grignard reagent ($RMgX$) is a compound which has a positive charge on the alkyl group and negative charge on the metal-halide group. It can be represented as follows:
\[{{R}^{-}}M{{g}^{+}}X\]
Grignard reagent is therefore highly reactive towards water molecules. Their reaction produces a hydrocarbon and magnesium hydroxy halide as shown below:
\[RMgX\text{ }+\text{ }{{H}_{2}}O\text{ }\xrightarrow{Dry\text{ Ether}}\text{ }R-H\text{ }+\text{ }Mg\left( OH \right)X\]
Hence anhydrous conditions are maintained so as to keep the Grignard reagent viable.
(iii) The structure of vinyl chloride and ethyl chloride are as follows:
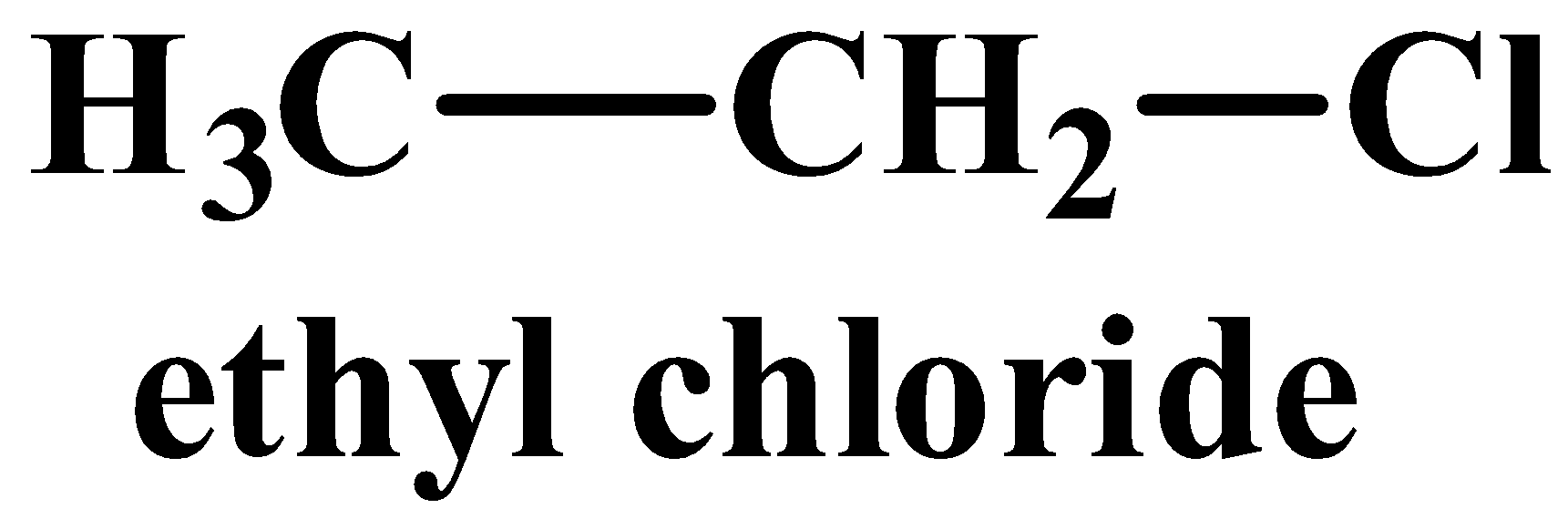
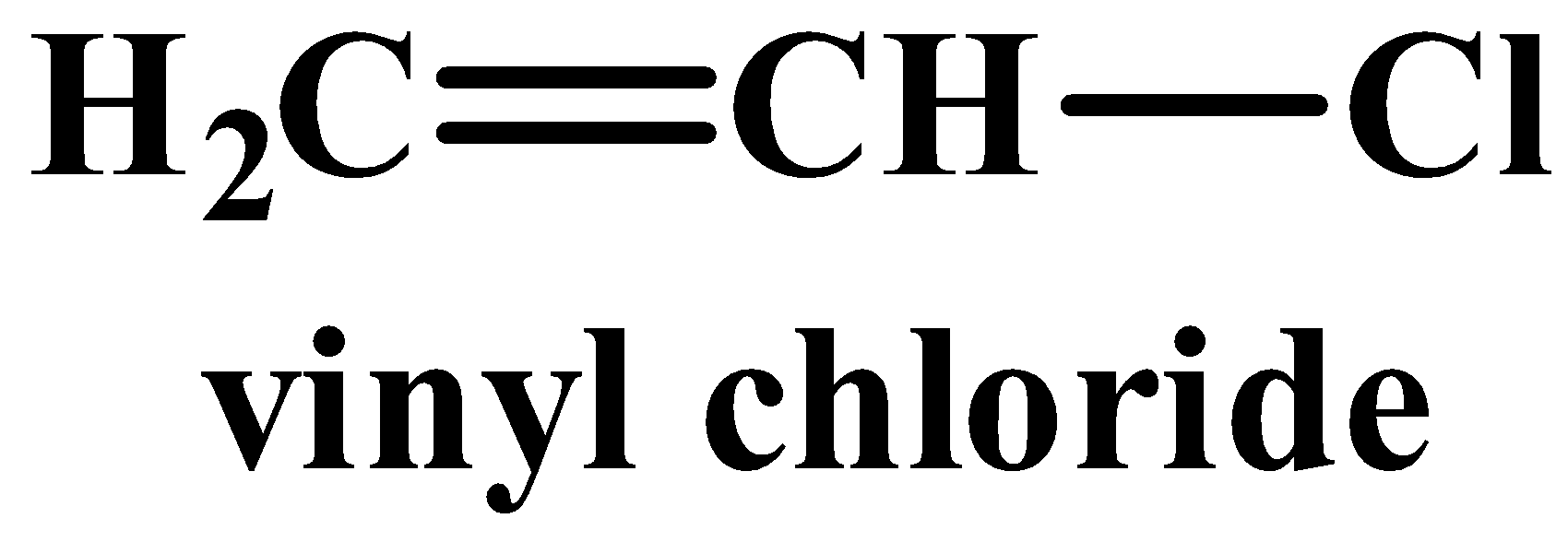
A nucleophilic reaction occurs when a nucleophile is the attacking reagent. A nucleophile has an excess of electrons and therefore favours attacking a substrate or molecule which is deficient in electrons. Out of the two compounds shown above, the vinyl chloride is unsaturated as it has a double bond. In this case, the hybridisation of the carbon atom with the double bong is $s{{p}^{2}}$, which means it has more “s” character implying it is more electronegative in nature. While all carbon atoms in ethyl chloride are $s{{p}^{3}}$hybridised and therefore lack a double bond. The nucleophile therefore favours ethyl chloride as it is more electron deficient than its counterpart.
Note: As a general rule of thumb, always try and remember that for a solute to be soluble in a required solvent, the solute-solvent interaction must be greater than both the solute-solute and solvent-solvent interactions.
Most of the reactions involving Grignard reagent require dry ether as a medium. Always remember to mention it while writing a chemical equation relating to this particular reagent.
An electrophile always favours an electron rich group while a nucleophile favours an electron deficient group.
Water is a proton donating solvent which hinders the existence of Grignard’s reagent.
Vinyl chloride’s structure contains a double bond whereas no such bond exists in ethyl chloride.
Complete step by step solution:
Let us go through the answer this question each part at a time:
(i) Alkyl halides are organic compounds. Water is a polar solvent having a high dielectric constant, which means it can separate charges from each other. This makes a compound dissolve in water. Compounds which are polar in nature are therefore soluble in water. Alkyl halides are polar only due to the halogen substituent. The alkyl part is hydrophobic in nature which repels water molecules. The bigger this hydrophobic part is the more insoluble that alkyl halide becomes. Hence, smaller alkyl halides are fairly soluble in water.
This phenomenon can be explained in yet another way. For a substance to dissolve in water, it has to form interactions with water molecules that are more stable than the interactions it already has. Only then there will be a net release of energy and the compound will dissolve. This will never happen in case of alkyl halides as:
- The covalent bonds are very strong and therefore not easy to break.
- As the hydrocarbon part of the alkyl halide is hydrophobic in nature, it will always repel water, making the new interaction weaker.
(ii) Grignard reagent ($RMgX$) is a compound which has a positive charge on the alkyl group and negative charge on the metal-halide group. It can be represented as follows:
\[{{R}^{-}}M{{g}^{+}}X\]
Grignard reagent is therefore highly reactive towards water molecules. Their reaction produces a hydrocarbon and magnesium hydroxy halide as shown below:
\[RMgX\text{ }+\text{ }{{H}_{2}}O\text{ }\xrightarrow{Dry\text{ Ether}}\text{ }R-H\text{ }+\text{ }Mg\left( OH \right)X\]
Hence anhydrous conditions are maintained so as to keep the Grignard reagent viable.
(iii) The structure of vinyl chloride and ethyl chloride are as follows:
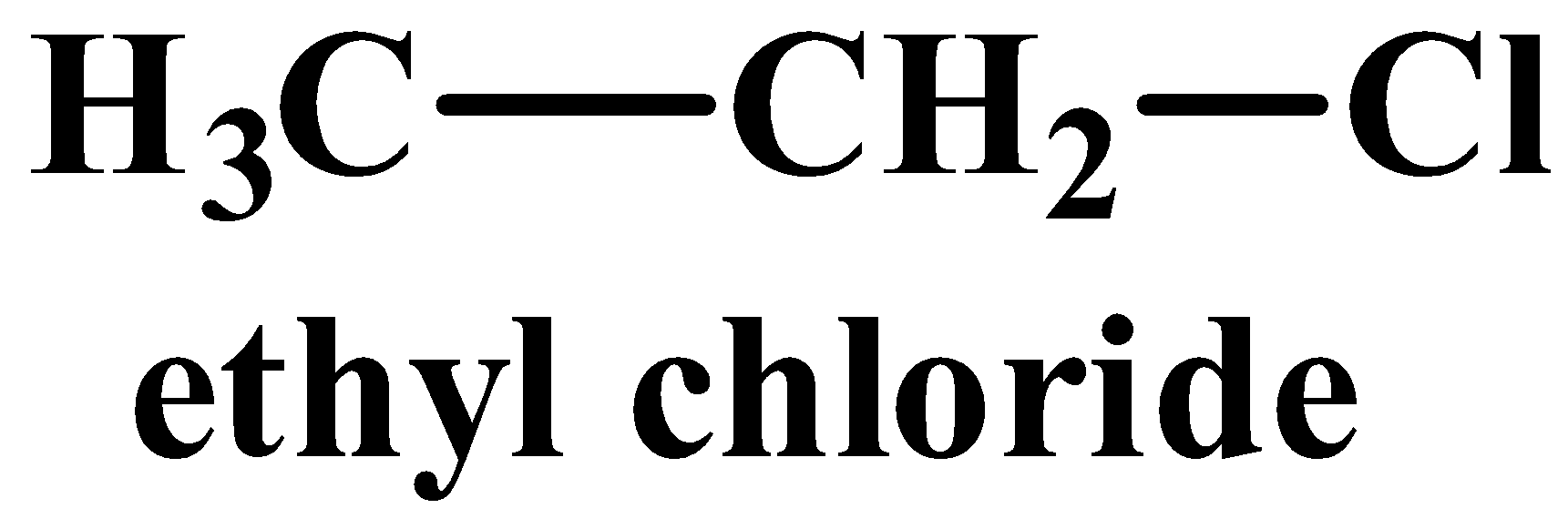
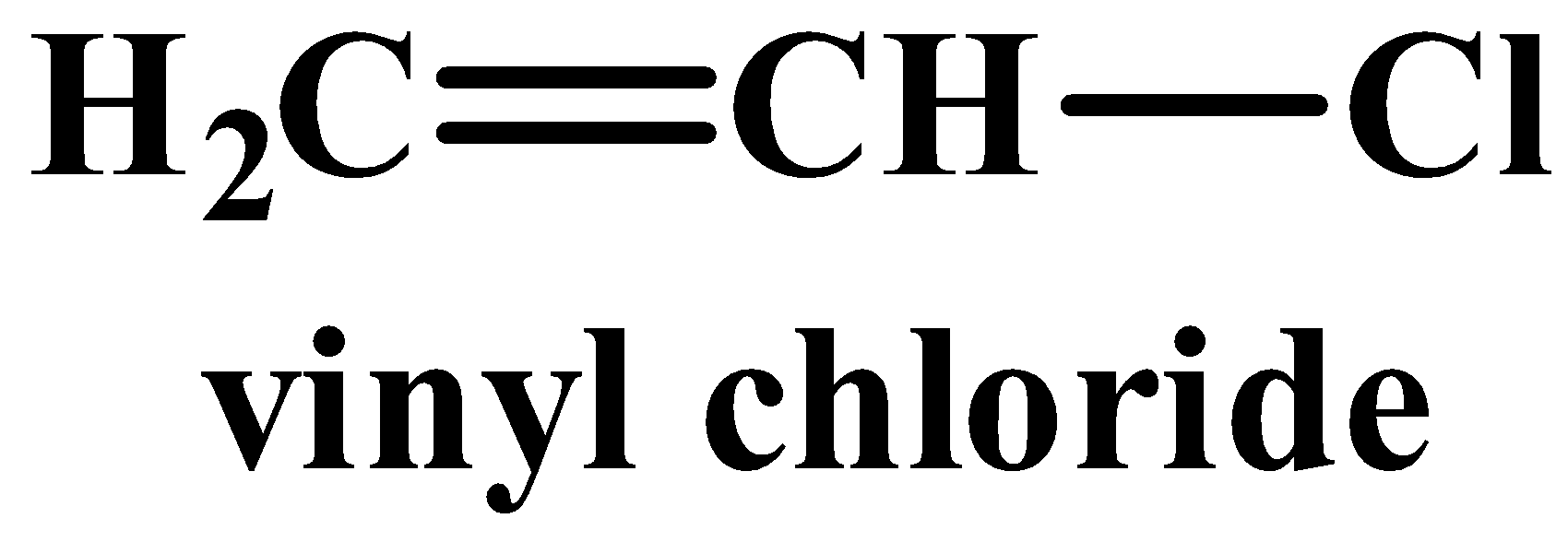
A nucleophilic reaction occurs when a nucleophile is the attacking reagent. A nucleophile has an excess of electrons and therefore favours attacking a substrate or molecule which is deficient in electrons. Out of the two compounds shown above, the vinyl chloride is unsaturated as it has a double bond. In this case, the hybridisation of the carbon atom with the double bong is $s{{p}^{2}}$, which means it has more “s” character implying it is more electronegative in nature. While all carbon atoms in ethyl chloride are $s{{p}^{3}}$hybridised and therefore lack a double bond. The nucleophile therefore favours ethyl chloride as it is more electron deficient than its counterpart.
Note: As a general rule of thumb, always try and remember that for a solute to be soluble in a required solvent, the solute-solvent interaction must be greater than both the solute-solute and solvent-solvent interactions.
Most of the reactions involving Grignard reagent require dry ether as a medium. Always remember to mention it while writing a chemical equation relating to this particular reagent.
An electrophile always favours an electron rich group while a nucleophile favours an electron deficient group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




