
Give reasons for the following:
(i) Aniline does not undergo Friedel-Crafts reaction.
(ii) \[{(C{H_3})_2}NH\] is more basic than \[{(C{H_3})_3}N\] in an aqueous solution.
(iii) Primary amines have higher boiling points than tertiary amines.
Answer
596.1k+ views
Hint:
Friedal-crafts reaction involves lewis acid as a catalyst. In aqueous conditions, there are two factors affecting the basicity of the amines, solvation and inductive effect. Hydrogen bonding between the atoms of the liquid has an effect on its boiling point.
Complete Step-by-Step Solution:
(i)
- Friedel-Crafts reaction uses a lewis acid as a catalyst. These lewis acids can be \[AlC{l_3},FeC{l_3},etc..\]
- Now Aniline has a nitrogen atom in its structure and it has a lone pair and it shows a basic character.
- So, acid-base type of reaction is always quick and will happen when Aniline and lewis acid will react.
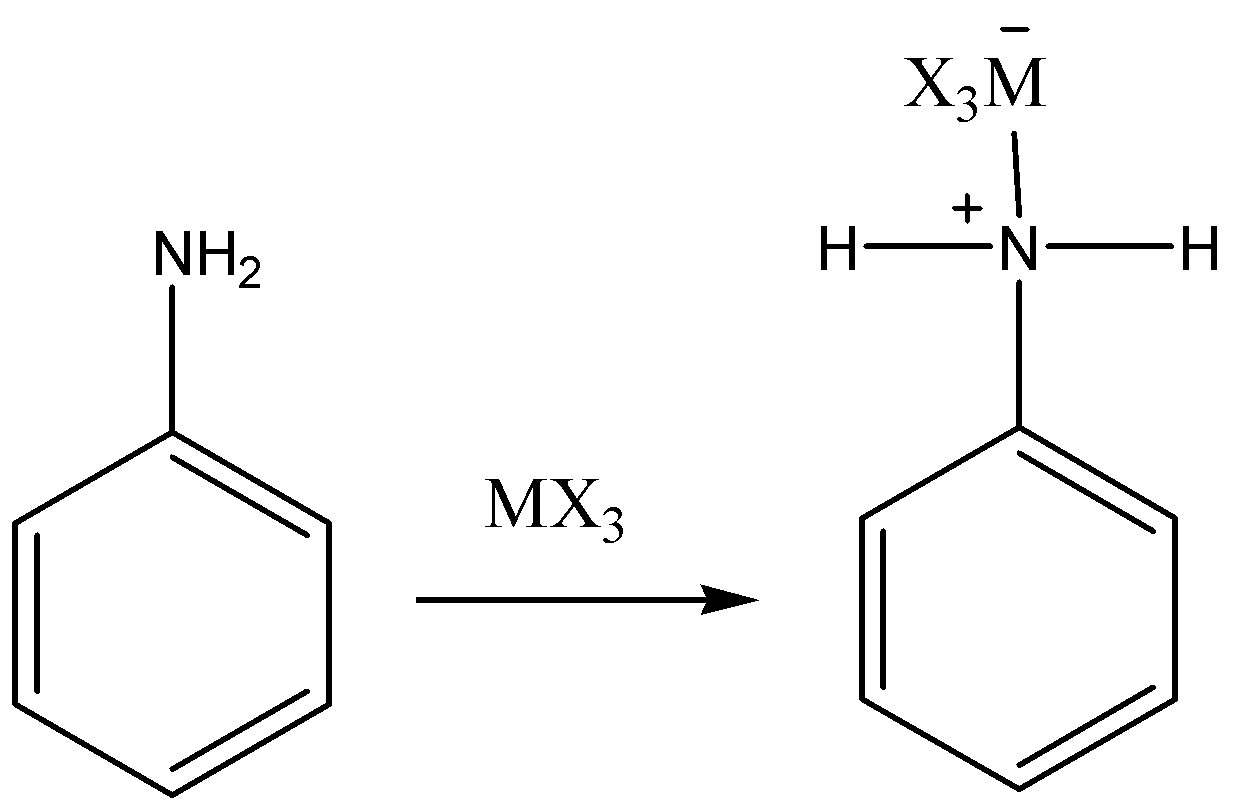
- So, given salt will be produced when an acid-base type of reaction will occur between aniline and a lewis acid. In this salt, the lone pair of aniline is donated and all the lewis acid molecules will form this type of salt with aniline, Hence, they will not be available for the catalysis and reaction will not occur further as there is no catalyst. Therefore Aniline will not give Friedal-Crafts reaction.
Thus, we can conclude that as anilne forms salt with the lewis acid in the Friedal-crafts reaction, it cannot give reaction further.
(ii)
When we compare the basicity of two amines in an aqueous solution, there are two factors present which can affect it. (1) Inductive effect and (2) solvation effect
Let’s examine \[{(C{H_3})_2}NH\] and \[{(C{H_3})_3}N\] with these factors.
- \[{(C{H_3})_2}NH\] has two alkyl groups bonded with nitrogen atom and \[{(C{H_3})_3}N\] has three alkyl groups bonded with nitrogen atom. So, the alkyl group has an electron donating inductive effect and hence it will increase the electron density on the nitrogen atom. We can say that lone pairs of \[{(C{H_3})_3}N\] will be more available for donation as nitrogen has high electron density.
- But there is also another factor affecting the basicity of amines in aqueous solutions is their solvation in solution. Let’s see how they will dissolve.
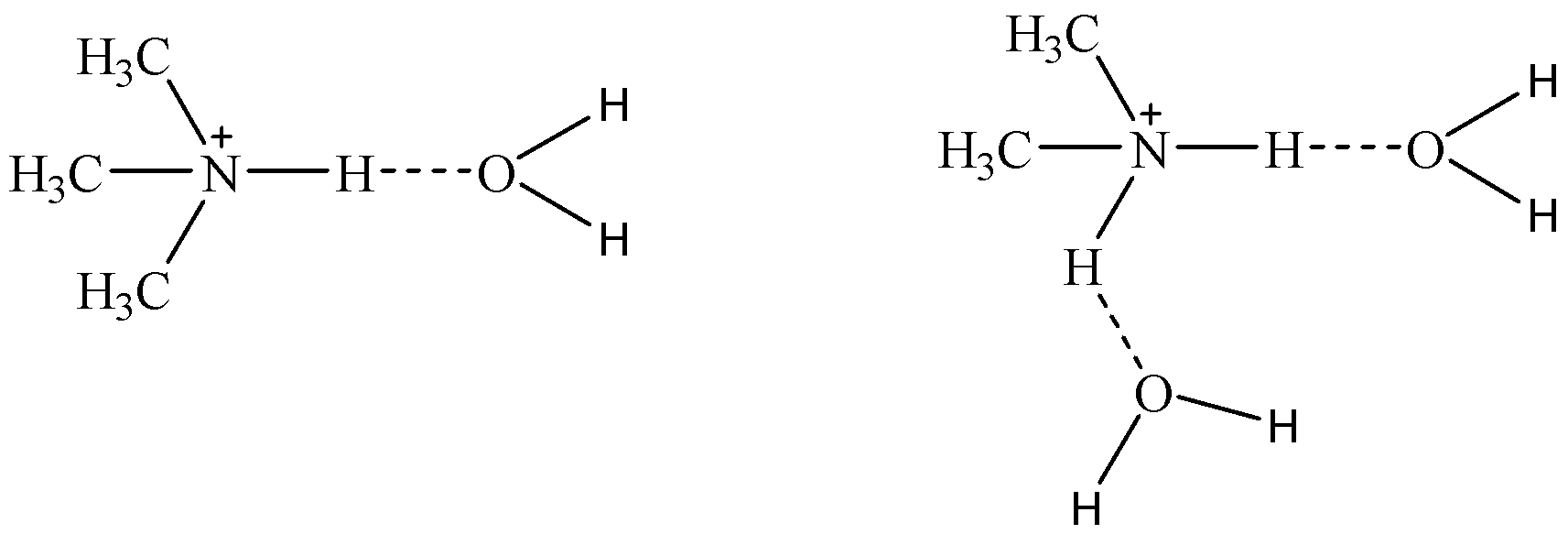
We can see that as Secondary ammonium salt has two polar hydrogen atoms, it can make hydrogen bonds with water and this will help in the solvation of amines.
- While tertiary amines have a polar hydrogen atom and hence it forms one hydrogen bond with a solvent molecule. So, solvation of tertiary amine means \[{(C{H_3})_3}N\] will be very less compared to \[{(C{H_3})_2}NH\].
Thus, In this case solvation effect has more influence on basicity than the inductive effect and hence \[{(C{H_3})_2}NH\] is more basic than \[{(C{H_3})_3}N\] in aqueous media.
(iii)
- Primary amines have two hydrogen atoms bonded with nitrogen atoms. These hydrogen atoms will make hydrogen bonding with other molecules of primary amines’ nitrogen atoms. This will increase attraction between two molecules of primary amine and that will result in higher boiling points.
- While tertiary amines do not have any hydrogen atom that can make hydrogen bonding with other atoms of tertiary amine. So, this will lead to less attraction between molecules of tertiary amines and this will result in lower boiling points of tertiary amines.
Hence, we can say that primary amines have higher boiling points than tertiary amines.
Note: Remember that acid base reactions are very fast and if there is any participating reaction that is occurring with acid-base type of reactions, then it will not dominate, that is the reason for aniline not giving Friedal-crafts reaction. Do not consider that tertiary amines will have higher boiling points than primary amines because of higher molecular weight because there are other factors also present affecting the boiling point.
Friedal-crafts reaction involves lewis acid as a catalyst. In aqueous conditions, there are two factors affecting the basicity of the amines, solvation and inductive effect. Hydrogen bonding between the atoms of the liquid has an effect on its boiling point.
Complete Step-by-Step Solution:
(i)
- Friedel-Crafts reaction uses a lewis acid as a catalyst. These lewis acids can be \[AlC{l_3},FeC{l_3},etc..\]
- Now Aniline has a nitrogen atom in its structure and it has a lone pair and it shows a basic character.
- So, acid-base type of reaction is always quick and will happen when Aniline and lewis acid will react.

- So, given salt will be produced when an acid-base type of reaction will occur between aniline and a lewis acid. In this salt, the lone pair of aniline is donated and all the lewis acid molecules will form this type of salt with aniline, Hence, they will not be available for the catalysis and reaction will not occur further as there is no catalyst. Therefore Aniline will not give Friedal-Crafts reaction.
Thus, we can conclude that as anilne forms salt with the lewis acid in the Friedal-crafts reaction, it cannot give reaction further.
(ii)
When we compare the basicity of two amines in an aqueous solution, there are two factors present which can affect it. (1) Inductive effect and (2) solvation effect
Let’s examine \[{(C{H_3})_2}NH\] and \[{(C{H_3})_3}N\] with these factors.
- \[{(C{H_3})_2}NH\] has two alkyl groups bonded with nitrogen atom and \[{(C{H_3})_3}N\] has three alkyl groups bonded with nitrogen atom. So, the alkyl group has an electron donating inductive effect and hence it will increase the electron density on the nitrogen atom. We can say that lone pairs of \[{(C{H_3})_3}N\] will be more available for donation as nitrogen has high electron density.
- But there is also another factor affecting the basicity of amines in aqueous solutions is their solvation in solution. Let’s see how they will dissolve.

We can see that as Secondary ammonium salt has two polar hydrogen atoms, it can make hydrogen bonds with water and this will help in the solvation of amines.
- While tertiary amines have a polar hydrogen atom and hence it forms one hydrogen bond with a solvent molecule. So, solvation of tertiary amine means \[{(C{H_3})_3}N\] will be very less compared to \[{(C{H_3})_2}NH\].
Thus, In this case solvation effect has more influence on basicity than the inductive effect and hence \[{(C{H_3})_2}NH\] is more basic than \[{(C{H_3})_3}N\] in aqueous media.
(iii)
- Primary amines have two hydrogen atoms bonded with nitrogen atoms. These hydrogen atoms will make hydrogen bonding with other molecules of primary amines’ nitrogen atoms. This will increase attraction between two molecules of primary amine and that will result in higher boiling points.
- While tertiary amines do not have any hydrogen atom that can make hydrogen bonding with other atoms of tertiary amine. So, this will lead to less attraction between molecules of tertiary amines and this will result in lower boiling points of tertiary amines.
Hence, we can say that primary amines have higher boiling points than tertiary amines.
Note: Remember that acid base reactions are very fast and if there is any participating reaction that is occurring with acid-base type of reactions, then it will not dominate, that is the reason for aniline not giving Friedal-crafts reaction. Do not consider that tertiary amines will have higher boiling points than primary amines because of higher molecular weight because there are other factors also present affecting the boiling point.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




