
Give reason: Graphite is used as a lubricant.
Answer
596.7k+ views
Hint: Attempt this question by stating the element from which graphite is made. The structure of graphite is responsible for the lubricity of it.
Complete step by step answer:
Graphite is an allotrope of carbon, which occurs as a soft, black, flaky solid. It is a lubricant and a moderate electrical conductor.
Graphite is also known by other common names, such as - black lead or plumbago.
Graphite is a crystalline form of the element carbon. All its atoms are \[s{{p}^{2}}\] hybridized. The atoms are arranged in a flat hexagonal structure, which is then layered in sheets. The individual layer of such hexagonal structures is known as graphene. This structural form is also known as the honeycomb lattice.
The structure of graphene is given below –
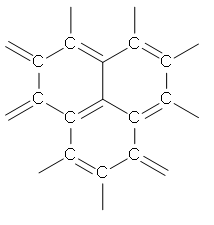
These graphene layers in graphite are bonded by Vander Waal forces. These forces are weak, which enable the graphene layers to slide over each other. This slippery nature of graphite is the reason for its lubricity.
Additional Information: Other than graphite, molybdenum disulfide is also used as a dry/solid lubricant. It is widely used in CV joints and space vehicles.
Note: Graphite, because of its loosely intact carbon atoms or free electrons, it can easily move around from one place to another. Therefore, graphite is a good conductor of electricity.
Complete step by step answer:
Graphite is an allotrope of carbon, which occurs as a soft, black, flaky solid. It is a lubricant and a moderate electrical conductor.
Graphite is also known by other common names, such as - black lead or plumbago.
Graphite is a crystalline form of the element carbon. All its atoms are \[s{{p}^{2}}\] hybridized. The atoms are arranged in a flat hexagonal structure, which is then layered in sheets. The individual layer of such hexagonal structures is known as graphene. This structural form is also known as the honeycomb lattice.
The structure of graphene is given below –

These graphene layers in graphite are bonded by Vander Waal forces. These forces are weak, which enable the graphene layers to slide over each other. This slippery nature of graphite is the reason for its lubricity.
Additional Information: Other than graphite, molybdenum disulfide is also used as a dry/solid lubricant. It is widely used in CV joints and space vehicles.
Note: Graphite, because of its loosely intact carbon atoms or free electrons, it can easily move around from one place to another. Therefore, graphite is a good conductor of electricity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




