
Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for 2-hydroxy-1,2,3-propane tricarboxylic acid.
Answer
564.3k+ views
Hint:We need to know what are condensed and bond line structural formulas and what functional groups are. Condensed formulas are the formulas in which the atoms are written in order. It is similar to a structural formula but written in a single line for the sake of convenience and to save space. On the other hand, bond line structural formula is a zigzag manner where every corner or terminal represents a methyl group. Functional groups are molecules responsible for their characteristic chemical reactions.
Complete step by step answer:
The given compound is 2-hydroxy-1,2,3-propane tricarboxylic acid.
1.Condensed formula: While complete structural formulas are written with the help of dashes which represent the covalent bonds, the condensed formula replaces the dashes by the groups attached to an atom by a subscript. Let us take the example of a simple molecule of ethane.
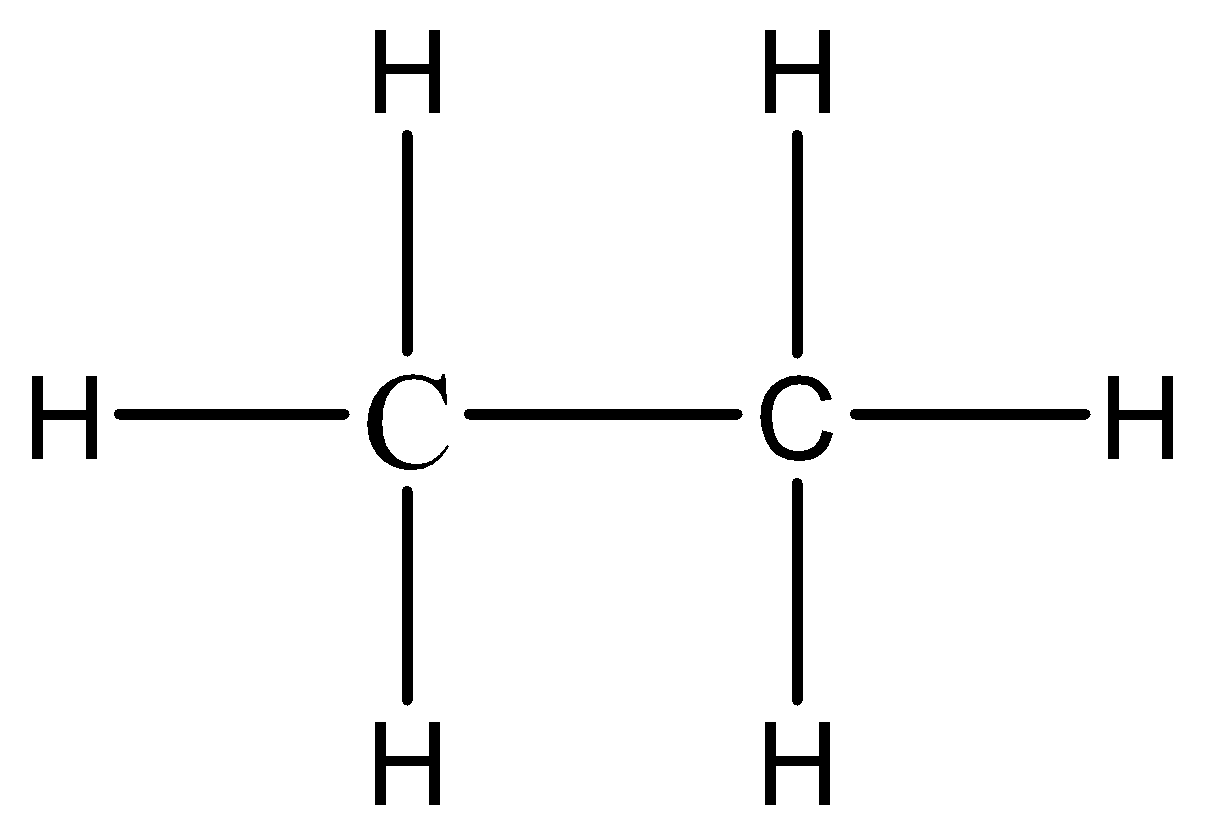
Complete structural formula of ethane.
The condensed formula of ethane will be \[C{H_3}C{H_3}\].
Therefore, the condensed formula of 2-hydroxy-1,2,3-propane tricarboxylic acid is : $\left( {COOH} \right)C{H_2}C\left( {OH} \right)\left( {COOH} \right)C{H_2}COOH$ .
2.Bond line structural formula: We must remember that the bond line structural formula is a zigzag representation where every corner represents a methyl group. For a molecule of ethane, the bond line structural formula will be a straight line with each terminal having a \[\;C{H_3}\] group.
Therefore the bond line structural formula of 2-hydroxy-1,2,3-propane tricarboxylic acid is :
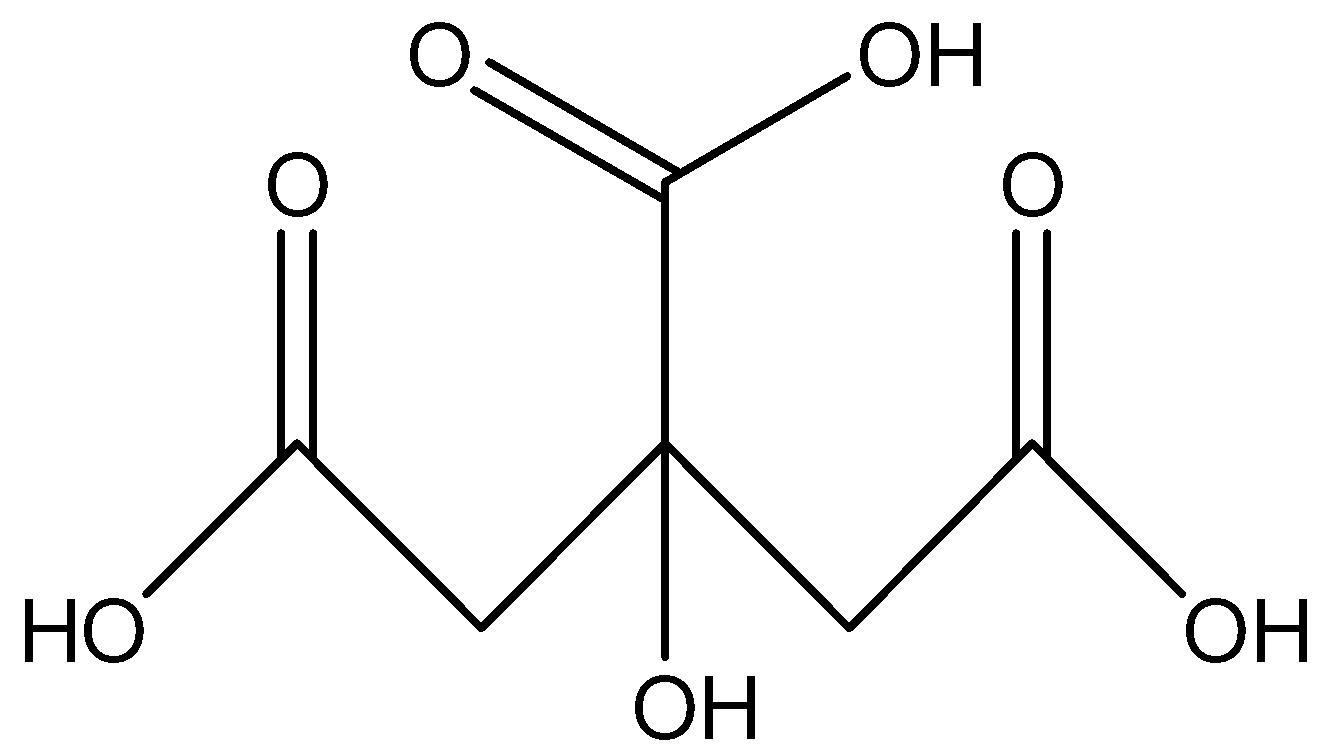
3.The functional groups present in 2-hydroxy-1,2,3-propane tricarboxylic acid are carboxylic acid ( \[ - COOH\] ) and alcoholic ( \[ - OH\] ) groups.
Note:
We have to note that these types of formulas are applicable to organic compounds only. They have only one chemical formula but can be structurally depicted in a number of ways. The bond line structural formula is also sometimes known as skeletal structure or skeletal formula. Also the molecular formula and condensed formula must not be confused. This can be explained with the example of ethane molecule whose molecular formula is represented as \[\;{C_2}{H_6}\] but the condensed formula is represented as \[C{H_3}C{H_3}\] .
Complete step by step answer:
The given compound is 2-hydroxy-1,2,3-propane tricarboxylic acid.
1.Condensed formula: While complete structural formulas are written with the help of dashes which represent the covalent bonds, the condensed formula replaces the dashes by the groups attached to an atom by a subscript. Let us take the example of a simple molecule of ethane.

Complete structural formula of ethane.
The condensed formula of ethane will be \[C{H_3}C{H_3}\].
Therefore, the condensed formula of 2-hydroxy-1,2,3-propane tricarboxylic acid is : $\left( {COOH} \right)C{H_2}C\left( {OH} \right)\left( {COOH} \right)C{H_2}COOH$ .
2.Bond line structural formula: We must remember that the bond line structural formula is a zigzag representation where every corner represents a methyl group. For a molecule of ethane, the bond line structural formula will be a straight line with each terminal having a \[\;C{H_3}\] group.
Therefore the bond line structural formula of 2-hydroxy-1,2,3-propane tricarboxylic acid is :

3.The functional groups present in 2-hydroxy-1,2,3-propane tricarboxylic acid are carboxylic acid ( \[ - COOH\] ) and alcoholic ( \[ - OH\] ) groups.
Note:
We have to note that these types of formulas are applicable to organic compounds only. They have only one chemical formula but can be structurally depicted in a number of ways. The bond line structural formula is also sometimes known as skeletal structure or skeletal formula. Also the molecular formula and condensed formula must not be confused. This can be explained with the example of ethane molecule whose molecular formula is represented as \[\;{C_2}{H_6}\] but the condensed formula is represented as \[C{H_3}C{H_3}\] .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




