
Give condensed and bond line structural formula and identify the functional group(s) present, if any, for :
2,2,4 - Trimethylpentane
Answer
588.6k+ views
Hint: Understand the IUPAC name given to the compound. The main carbon chain is going to consist of 5 carbon atoms as it is pentane. Now draw the substituent carbon groups. While writing in condensed form, write the substituent groups in brackets.
Complete step by step answer:
We have already identified the main chain to be consisting of 5 carbon atoms.
So, the skeleton of the organic compound looks like:
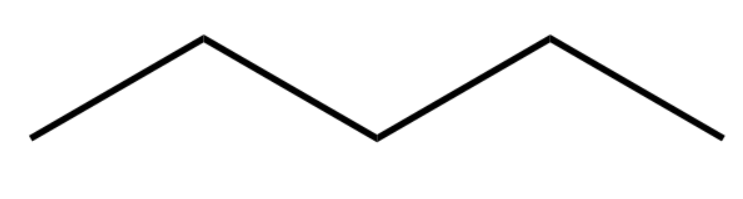
Now we identify three substituent carbon groups (methyl groups) attached to the 2nd carbon atom and the 4th carbon atom. Since there is no functional group, we can start counting from either of the sides.
The final bond line structural formula looks like:
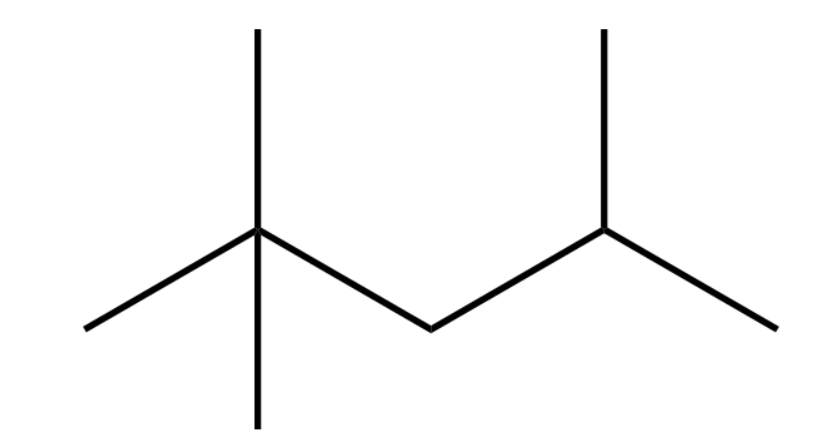
The substituent groups are written in the brackets while writing the compound name structure in condensed form:
Formula: ${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{2}}}\text{CHC}{{\text{H}}_{\text{2}}}\text{C(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}$
The above organic compound does not have any functional group attached to the carbon atom.
Additional Information:
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich, Switzerland and its administrative office is called IUPAC secretariat.
Note: In the above question it was easy to choose the longest carbon chain and the main functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:
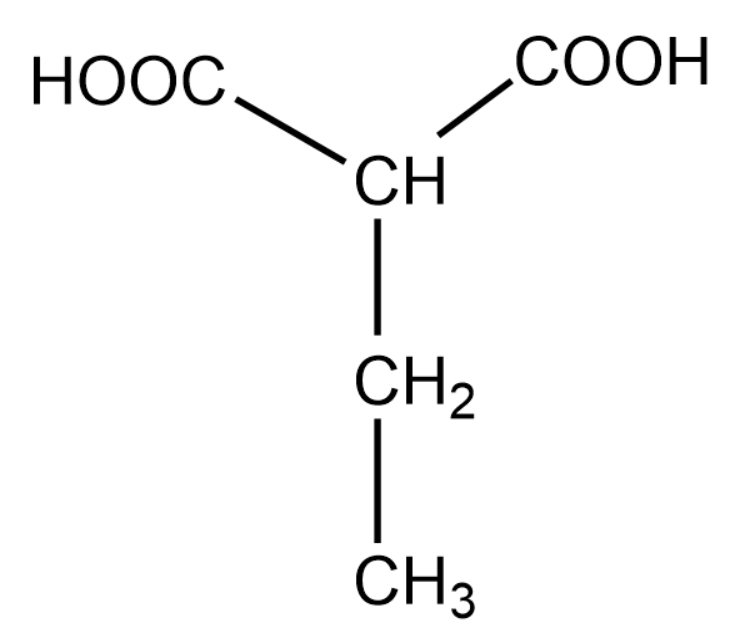
For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
Complete step by step answer:
We have already identified the main chain to be consisting of 5 carbon atoms.
So, the skeleton of the organic compound looks like:

Now we identify three substituent carbon groups (methyl groups) attached to the 2nd carbon atom and the 4th carbon atom. Since there is no functional group, we can start counting from either of the sides.
The final bond line structural formula looks like:

The substituent groups are written in the brackets while writing the compound name structure in condensed form:
Formula: ${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{2}}}\text{CHC}{{\text{H}}_{\text{2}}}\text{C(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}$
The above organic compound does not have any functional group attached to the carbon atom.
Additional Information:
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich, Switzerland and its administrative office is called IUPAC secretariat.
Note: In the above question it was easy to choose the longest carbon chain and the main functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:

For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




