
Give any two differences between alkene and alkane.
Answer
537.6k+ views
Hint :Both alkene and alkane are hydrocarbons. All the organic compounds possessing carbon as well as hydrogen atoms are known as hydrocarbons. Hydrocarbons are naturally-occurring compounds and they generally form the basis of natural gas, crude oil, coal, and other energy sources.
Complete Step By Step Answer:Hydrocarbons are mainly classified as saturated hydrocarbons and unsaturated hydrocarbons. Now, let us look at the differences between alkenes and alkanes as listed in the table below:
Note :
If hydrocarbons enter the lungs, it usually causes pneumonia and even death. Some hydrocarbons can also cause coma, irregular heart rhythms, seizures, or damage to the liver or kidneys.
Complete Step By Step Answer:Hydrocarbons are mainly classified as saturated hydrocarbons and unsaturated hydrocarbons. Now, let us look at the differences between alkenes and alkanes as listed in the table below:
| S.No. | Alkane | Alkene |
| 1. | Alkanes are saturated hydrocarbons consisting of only single bonded carbon atoms. They are known as saturated owing to the capacity of each carbon atom to form bond with as many hydrogen atoms as possible i.e. saturated with the hydrogen atoms. | Alkenes are unsaturated hydrocarbons which possess double covalent bonds between the adjacent carbon atoms. The term "unsaturated" indicates that more hydrogen atoms can be appended into the hydrocarbon in order to make it saturated. |
| 2. | They are less reactive in nature. | They are reactive in nature. |
| 3. | The general formula of alkane is $ {C_n}{H_{2n + 2}} $ | The general formula of alkene is $ {C_n}{H_{2n}} $ (excluding cyclic compounds) |
| 4. | Examples of alkane are methane, ethane, propane, etc. The chemical structure of propane having chemical formula of $ {C_3}{H_8} $ is depicted below: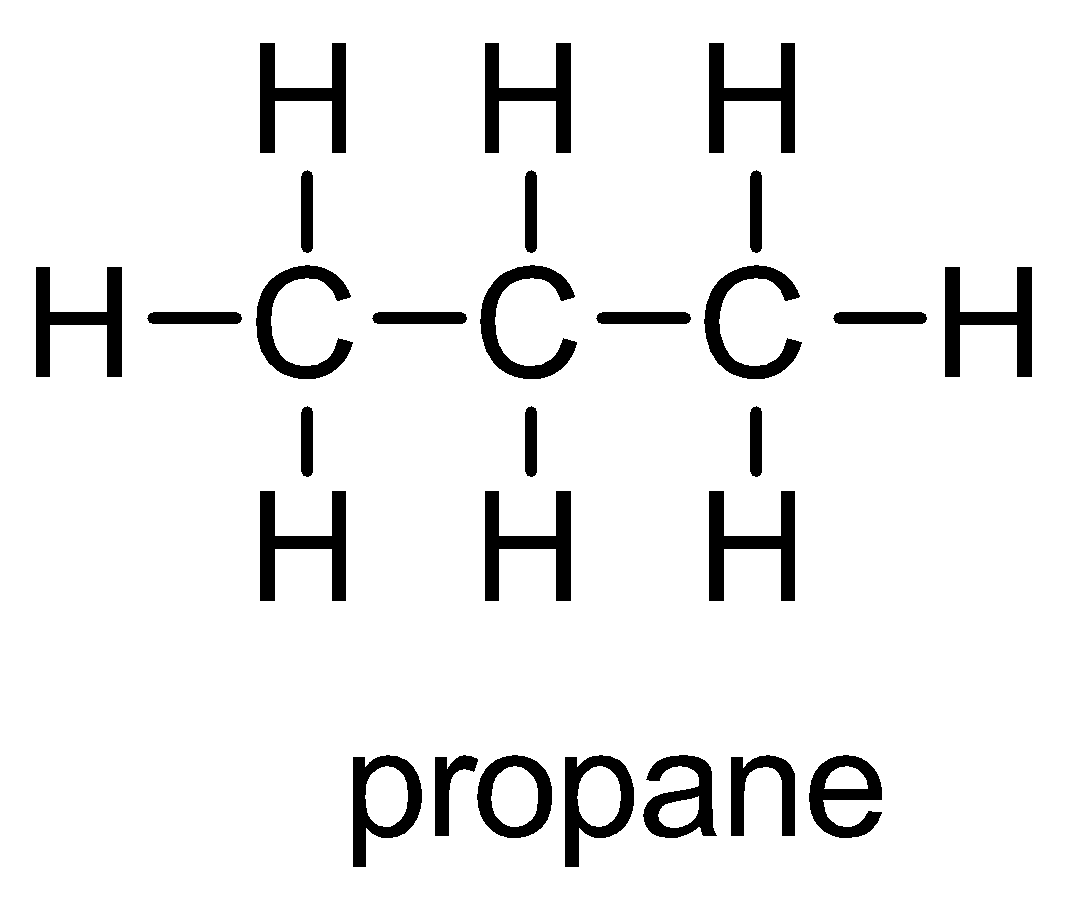
| Examples of alkene are propene, cis-2-butene, pentene, etc. The chemical structure of propene having a chemical formula of $ {C_3}{H_6} $ is depicted below: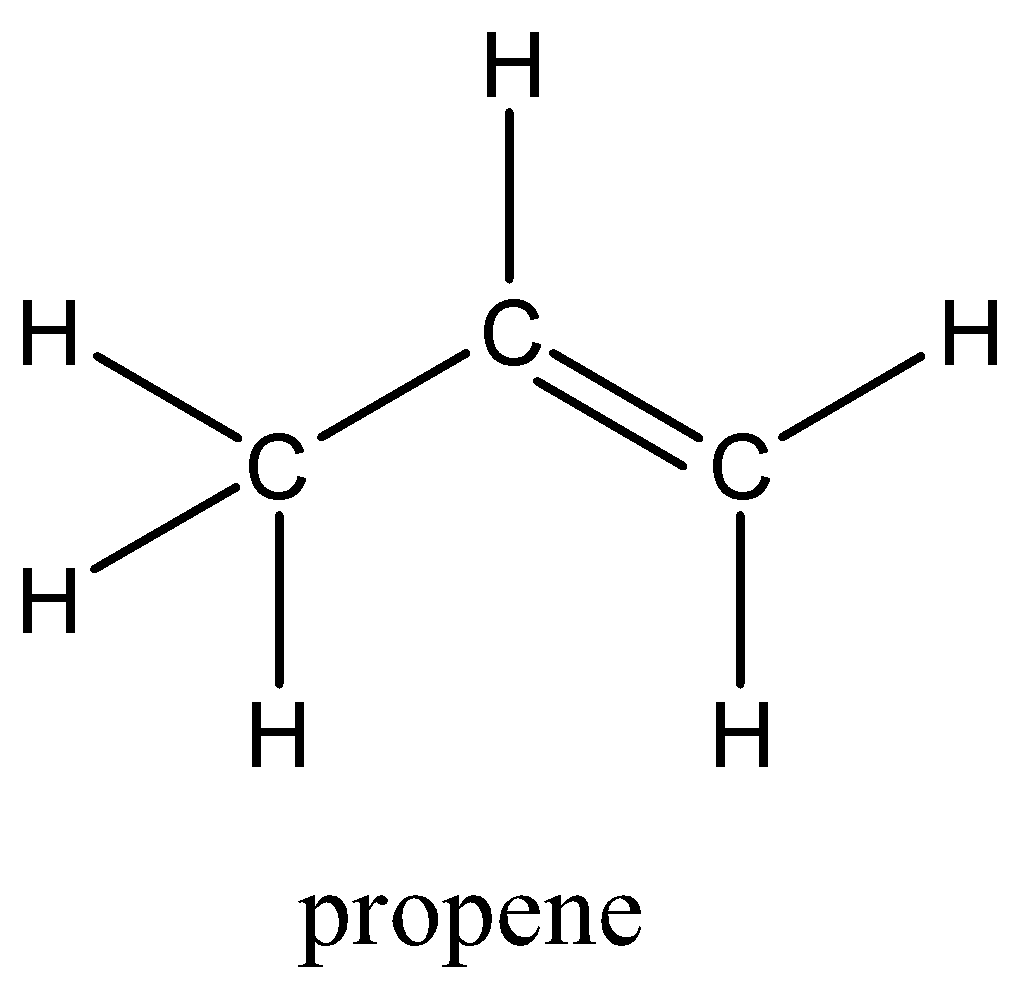
|
Note :
If hydrocarbons enter the lungs, it usually causes pneumonia and even death. Some hydrocarbons can also cause coma, irregular heart rhythms, seizures, or damage to the liver or kidneys.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




