
Give a structure of \[C{{r}_{2}}{{O}_{7}}^{2-}\] ion.
Answer
570.9k+ views
Hint: Oxygen generally has an oxidation state of $-2$, as it has six electrons in its outermost orbitals, and it needs only two, in order to attain stable octet configuration, so it gains two electrons which gives it that charge.
The species which have net negative charge are termed as anions, and the ones which have high oxygen concentration are best known for their oxidising properties.
Complete step by step answer:
In order to draw the structure of dichromate ions, we need to find out the number of bonds which are being formed between the chromium and oxygen atoms and justify the net charge of the ion.
We know that oxygen contains six electrons in its outermost orbital and it gains two electrons in order to attain a stable octet configuration.
So, it usually has $-2$ charge in it, or in other words its oxidation state is negative two. The negative sign indicates that the electron is being gained and not lost.
The species which lose electrons in order to attain stable octet configuration, has a positive charge, or a positive oxidation state to express that they have lost electrons.
Now, as we can see in the chemical formula of dichromate, there are seven such oxygen atoms attached to, two of the chromium atoms. Now we know that the chromium atoms have six electrons in the outermost orbitals, and it could lose all those electrons to oxygen, in order to form bonds with oxygen atoms.
Now if we consider the structure of dichromate, keeping this concept in mind, the structure would look like the following diagram,
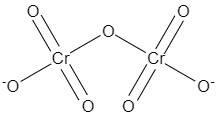
Now as we can see four of these oxygens are attached to the two chromium atoms through double bonds, and one oxygen is acting like a bridge between the two chromium atoms, whereas rest of the two oxygens satisfy their one valency by forming a bond with chromium, and one of the electron remains unshared in both of the oxygens.
Note: The structure of dichromate consists of seven oxygens, where one acts like a bridge between two chromium atoms, and the rest of the oxygens share the bond with the chromium.
However two of the oxygen doesn’t get to share both of its electrons as the chromium already attains its stable octet configuration, so these two oxygens have negative charge in it, which results in a net charge of $-2$ in the ion.
The species which have net negative charge are termed as anions, and the ones which have high oxygen concentration are best known for their oxidising properties.
Complete step by step answer:
In order to draw the structure of dichromate ions, we need to find out the number of bonds which are being formed between the chromium and oxygen atoms and justify the net charge of the ion.
We know that oxygen contains six electrons in its outermost orbital and it gains two electrons in order to attain a stable octet configuration.
So, it usually has $-2$ charge in it, or in other words its oxidation state is negative two. The negative sign indicates that the electron is being gained and not lost.
The species which lose electrons in order to attain stable octet configuration, has a positive charge, or a positive oxidation state to express that they have lost electrons.
Now, as we can see in the chemical formula of dichromate, there are seven such oxygen atoms attached to, two of the chromium atoms. Now we know that the chromium atoms have six electrons in the outermost orbitals, and it could lose all those electrons to oxygen, in order to form bonds with oxygen atoms.
Now if we consider the structure of dichromate, keeping this concept in mind, the structure would look like the following diagram,

Now as we can see four of these oxygens are attached to the two chromium atoms through double bonds, and one oxygen is acting like a bridge between the two chromium atoms, whereas rest of the two oxygens satisfy their one valency by forming a bond with chromium, and one of the electron remains unshared in both of the oxygens.
Note: The structure of dichromate consists of seven oxygens, where one acts like a bridge between two chromium atoms, and the rest of the oxygens share the bond with the chromium.
However two of the oxygen doesn’t get to share both of its electrons as the chromium already attains its stable octet configuration, so these two oxygens have negative charge in it, which results in a net charge of $-2$ in the ion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




