
What functional groups are present in acetaminophen?
Answer
515.4k+ views
Hint: A functional group in organic chemistry is a distinct group of atoms or bonds within a compound that is responsible for the compound's characteristic chemical reactions. Regardless of the compound in which it is found, the same functional group can behave similarly by undergoing identical reactions.
Complete answer:
Acetaminophen, also known as paracetamol, is a drug that is used to relieve fever and mild to severe discomfort. Paracetamol only marginally lowers body temperature at a normal dose; it is inferior to ibuprofen in this regard, and the effects of its use for fever remain uncertain. In acute migraine, paracetamol greatly relieves discomfort, but just marginally in episodic anxiety headache. The aspirin/paracetamol/caffeine mixture, on the other hand, assists in all symptoms and is used as a first-line therapy. While paracetamol is useful for post-surgical pain, it falls short of ibuprofen.
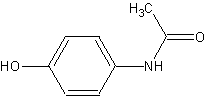
The functional groups present are
A hydroxyl group is located at the top of the molecule. It's easy to refer to it as an alcohol group.
An –OH group connected to a benzene ring, on the other hand, has unique properties. It is also known as a phenol group or a phenolic OH.
The aromatic ring has six members.
The bottom of the molecule contains a mono substituted or secondary amide group.
An amide's general formula is\[RCONH{R_2}\]. All of the R groups on the N atom in this case is a hydrogen atom.
Note:
Paracetamol was invented in 1877. It is the most widely used pain and fever drug in both the United States and Europe. It is on the WHO's List of Essential Medicines. Paracetamol is available as a generic drug, as well as under brand names such as Tylenol and Panadol. For over 27 million prescriptions in 2018, it was the eighteenth most widely used drug in the United States in 2018.
Complete answer:
Acetaminophen, also known as paracetamol, is a drug that is used to relieve fever and mild to severe discomfort. Paracetamol only marginally lowers body temperature at a normal dose; it is inferior to ibuprofen in this regard, and the effects of its use for fever remain uncertain. In acute migraine, paracetamol greatly relieves discomfort, but just marginally in episodic anxiety headache. The aspirin/paracetamol/caffeine mixture, on the other hand, assists in all symptoms and is used as a first-line therapy. While paracetamol is useful for post-surgical pain, it falls short of ibuprofen.

The functional groups present are
A hydroxyl group is located at the top of the molecule. It's easy to refer to it as an alcohol group.
An –OH group connected to a benzene ring, on the other hand, has unique properties. It is also known as a phenol group or a phenolic OH.
The aromatic ring has six members.
The bottom of the molecule contains a mono substituted or secondary amide group.
An amide's general formula is\[RCONH{R_2}\]. All of the R groups on the N atom in this case is a hydrogen atom.
Note:
Paracetamol was invented in 1877. It is the most widely used pain and fever drug in both the United States and Europe. It is on the WHO's List of Essential Medicines. Paracetamol is available as a generic drug, as well as under brand names such as Tylenol and Panadol. For over 27 million prescriptions in 2018, it was the eighteenth most widely used drug in the United States in 2018.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




