
From the following reactions, identify the reaction that gives carboxylic acids as products.
A. $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[2.Dil.{{H}_{2}}S{{O}_{4}}]{1.KMn{{O}_{4}}/KOH}$
B.
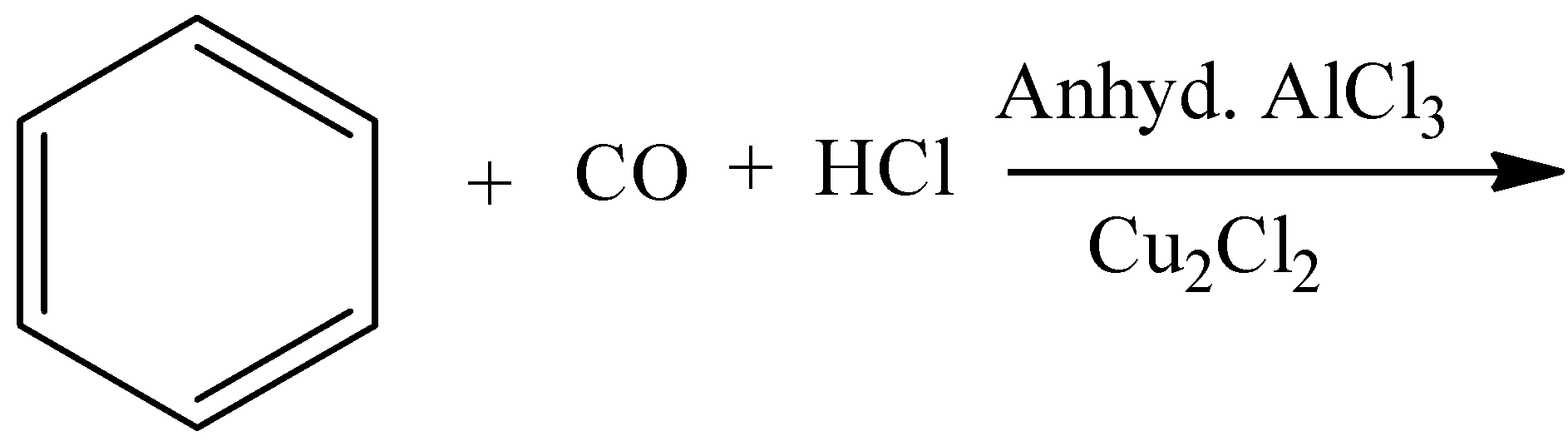
C.
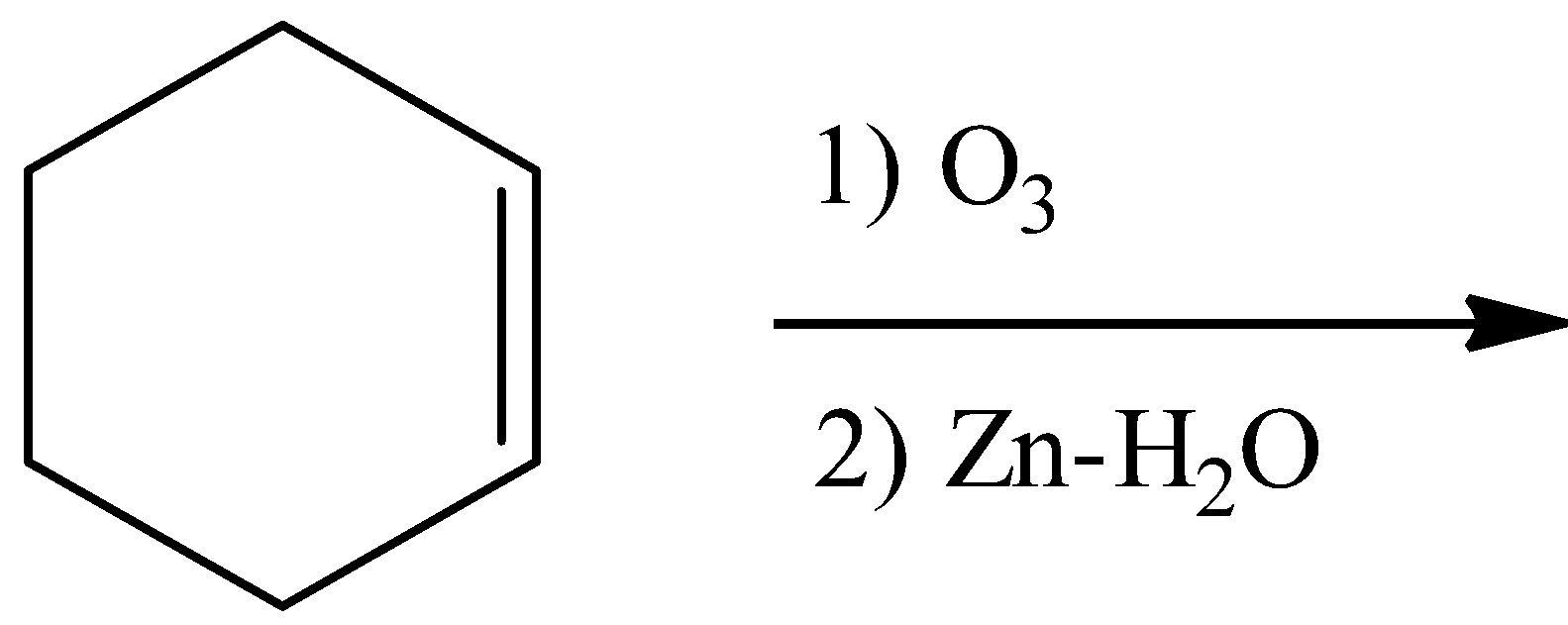
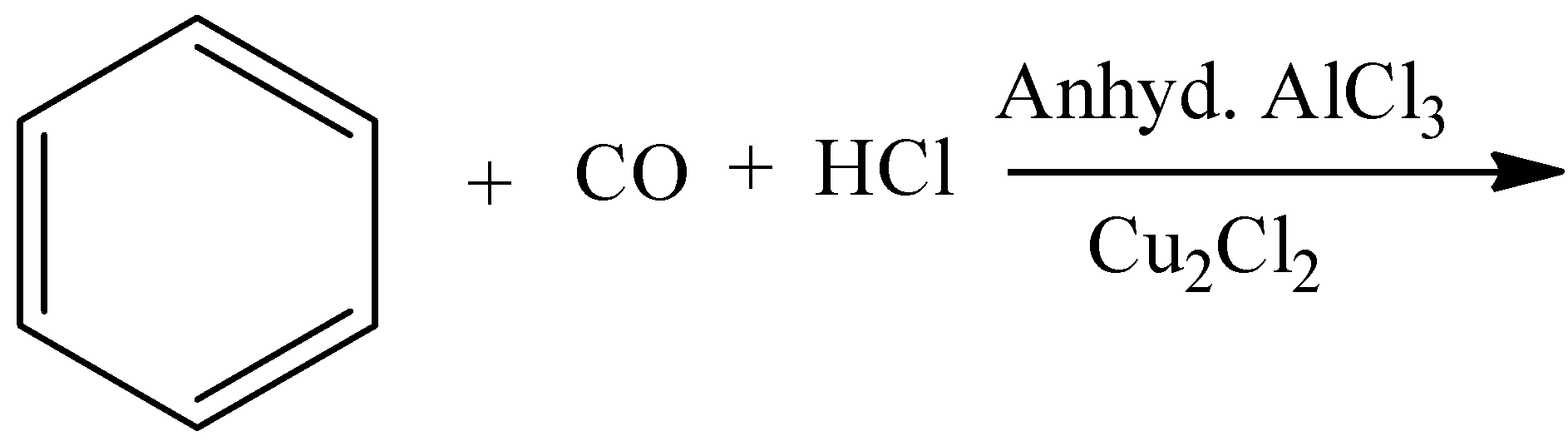
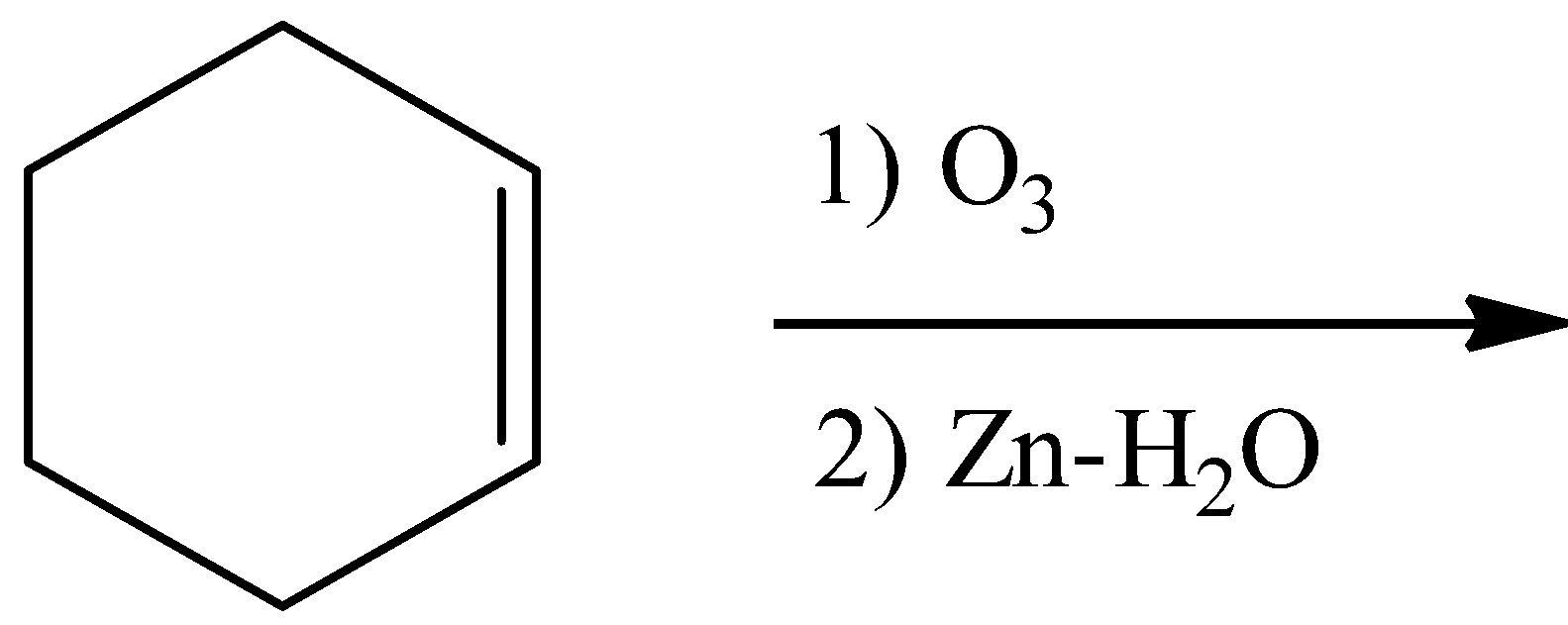
Answer
563.1k+ views
Hint: The functional group –COOH is called carboxylic acid functional group. Generally we need a strong oxidizing agent to prepare carboxylic acids from the reactants having different functional groups.
Complete step by step answer:
- In the question it is asked that among the given chemical reactions, identify the chemical reaction which will give carboxylic acid as the product.
- Coming to option A,
$C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[2.Dil.{{H}_{2}}S{{O}_{4}}]{1.KMn{{O}_{4}}/KOH}$
- Generally primary alcohols react with strong oxidizing agents like potassium permanganate in the presence of an acid that produces carboxylic acid as the products.
$C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[2.Dil.{{H}_{2}}S{{O}_{4}}]{1.KMn{{O}_{4}}/KOH}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH$
- Therefore butanol reacts with potassium permanganate in presence of dilute sulphuric acid and produces butanoic acid as the product.
- Coming to option B,
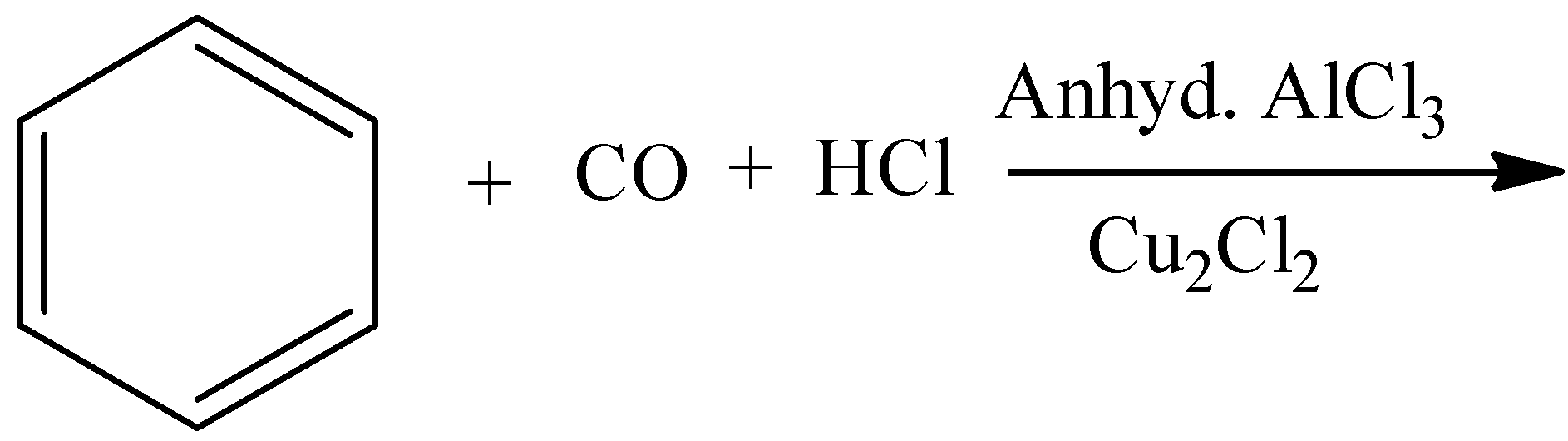
- In the above chemical reaction there is no strong oxidizing agent and there is no reactant which gives acid on oxidation.
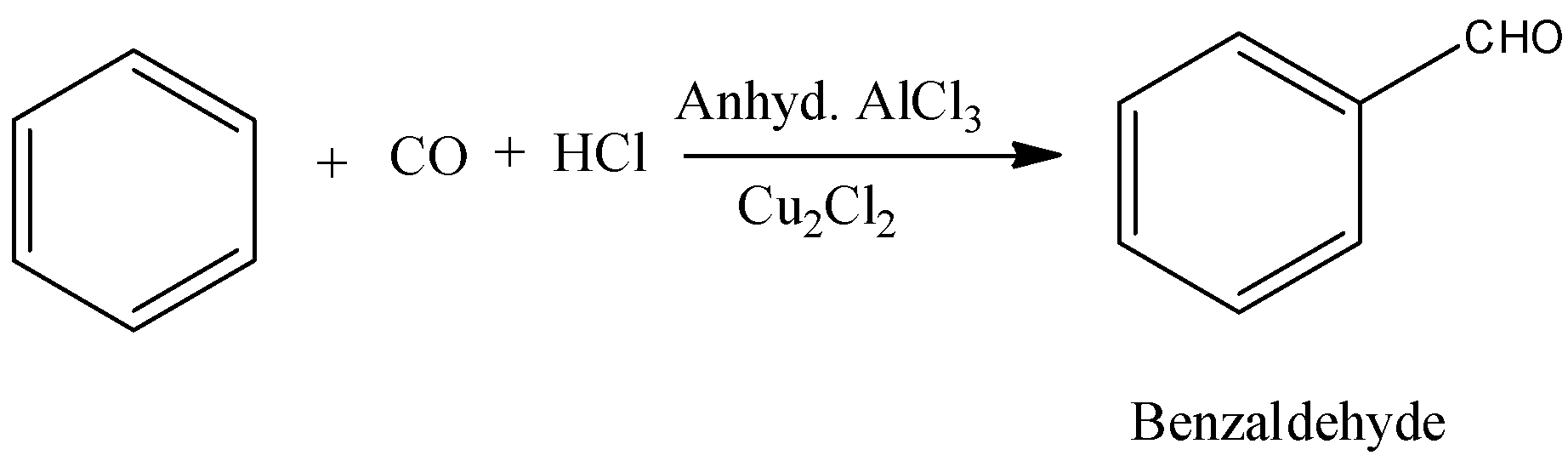
- The product formed in the above chemical reaction is an aldehyde.
- So, option B is wrong.
- Coming to option C,
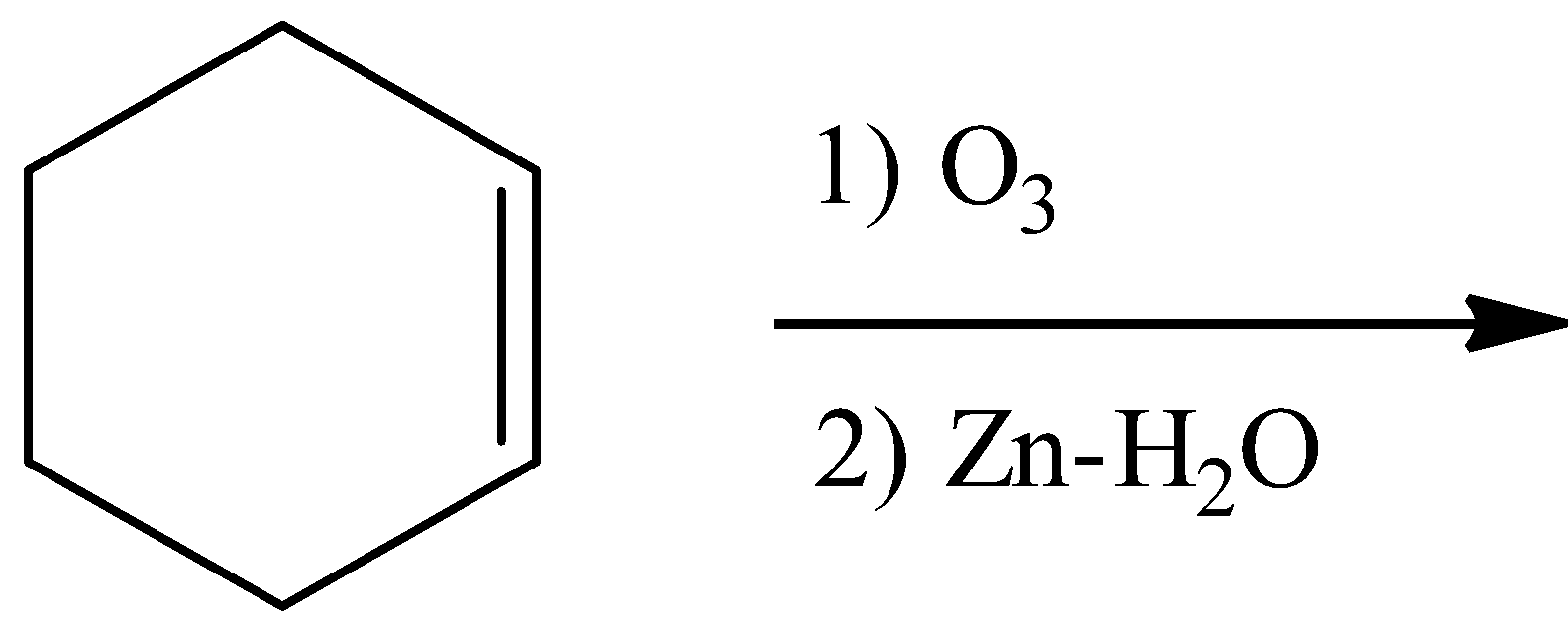
- Ozonolysis of the double bond produces either ketones or aldehydes as the products.
- So, the product in the chemical reaction in option C is as follows.
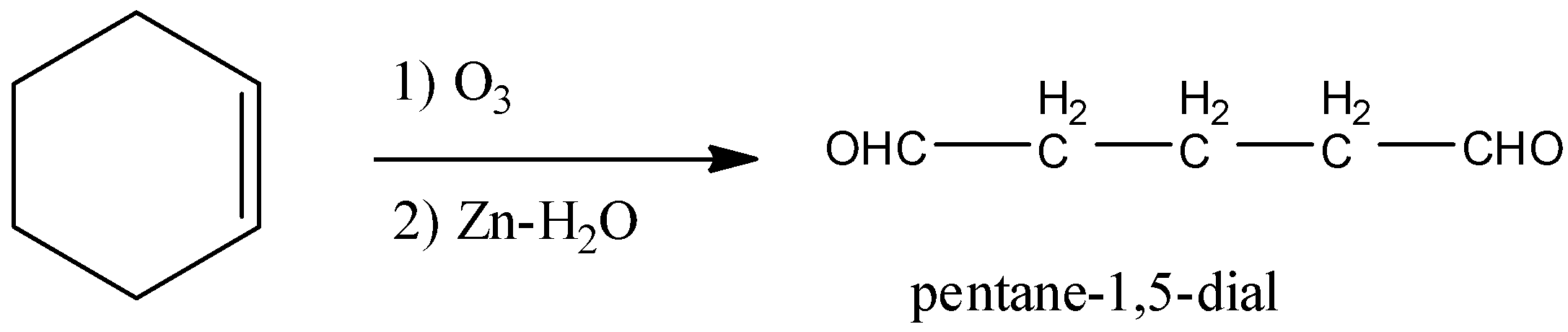
- So option C is also wrong.
- Therefore primary alcohols will give carboxylic acids on oxidation with strong oxidizing agents. The correct option is option “A” .
Note: The formation of benzaldehyde by using benzene, carbon monoxide and hydrochloric acid is called Gattermann Koch reaction. In this reaction aluminum trichloride is going to act as a catalyst. This reaction is an example of electrophilic substitution reaction.
Complete step by step answer:
- In the question it is asked that among the given chemical reactions, identify the chemical reaction which will give carboxylic acid as the product.
- Coming to option A,
$C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[2.Dil.{{H}_{2}}S{{O}_{4}}]{1.KMn{{O}_{4}}/KOH}$
- Generally primary alcohols react with strong oxidizing agents like potassium permanganate in the presence of an acid that produces carboxylic acid as the products.
$C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[2.Dil.{{H}_{2}}S{{O}_{4}}]{1.KMn{{O}_{4}}/KOH}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH$
- Therefore butanol reacts with potassium permanganate in presence of dilute sulphuric acid and produces butanoic acid as the product.
- Coming to option B,
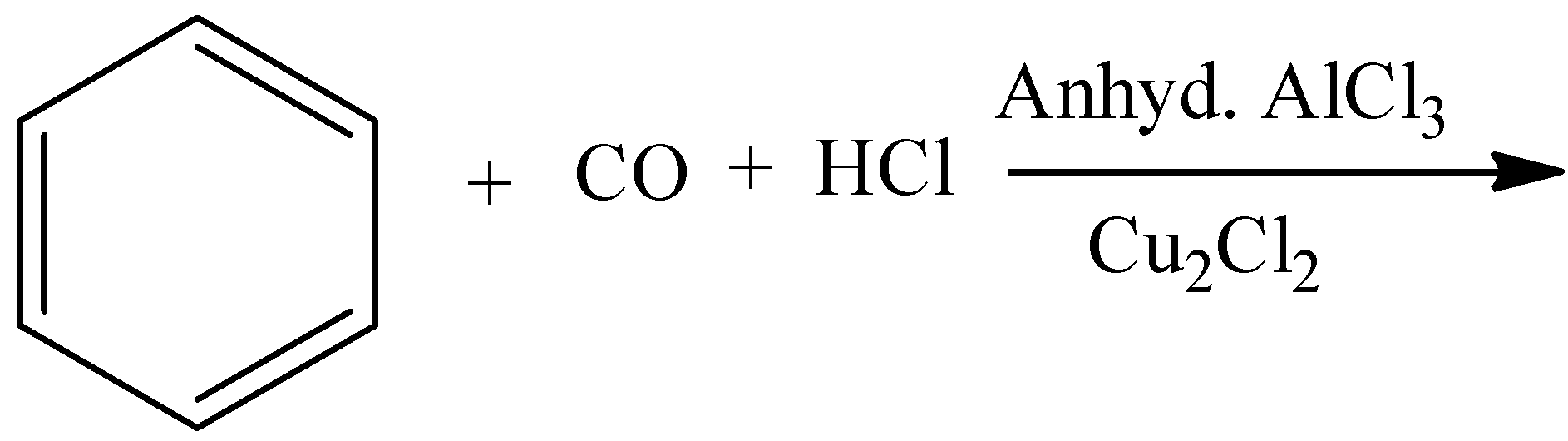
- In the above chemical reaction there is no strong oxidizing agent and there is no reactant which gives acid on oxidation.
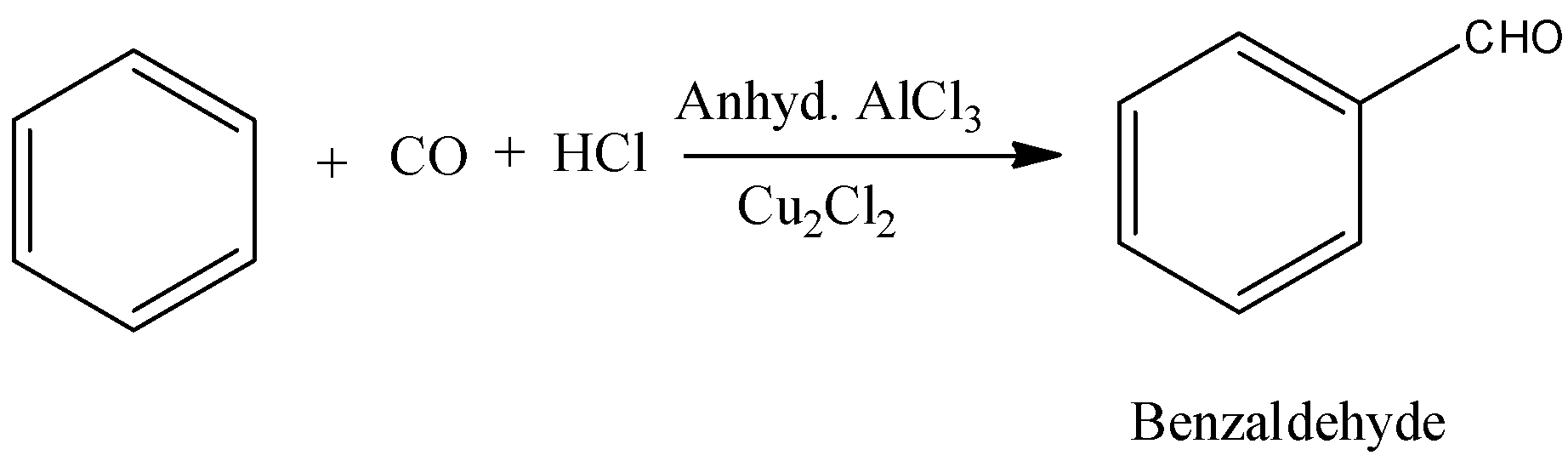
- The product formed in the above chemical reaction is an aldehyde.
- So, option B is wrong.
- Coming to option C,
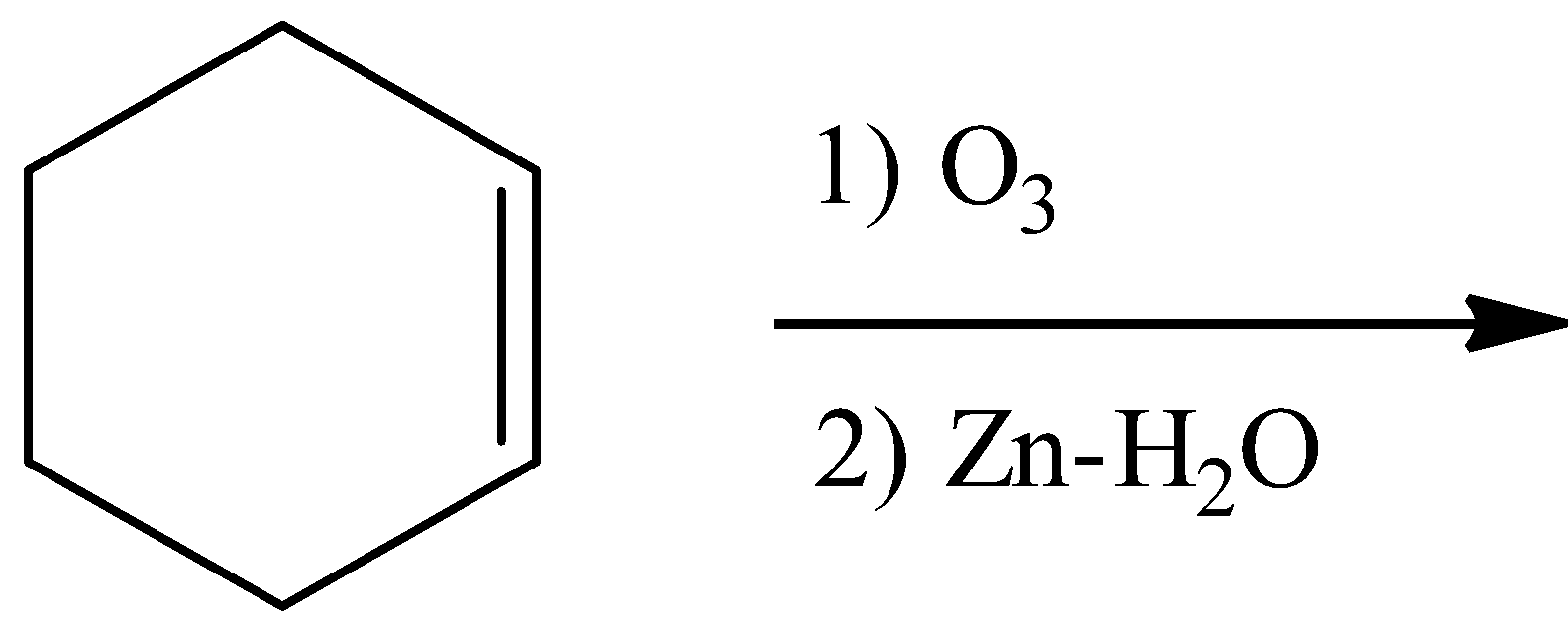
- Ozonolysis of the double bond produces either ketones or aldehydes as the products.
- So, the product in the chemical reaction in option C is as follows.

- So option C is also wrong.
- Therefore primary alcohols will give carboxylic acids on oxidation with strong oxidizing agents. The correct option is option “A” .
Note: The formation of benzaldehyde by using benzene, carbon monoxide and hydrochloric acid is called Gattermann Koch reaction. In this reaction aluminum trichloride is going to act as a catalyst. This reaction is an example of electrophilic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE

Calculate the equivalent resistance between a and b class 12 physics CBSE

How many states of matter are there in total class 12 chemistry CBSE

Which of the following is the best conductor of electricity class 12 physics CBSE




