
Friedel-Craft’s reaction using $MeCl$ and anhydrous $AlC{l_3}$ will take place most efficiently with:
(A) benzene
(B) nitrobenzene
(C) acetophenone
(D) toluene
Answer
578.1k+ views
Hint: Friedel-Craft’s is an electrophilic aromatic substitution reaction in which a carbocation attacks on an aromatic ring.
There are two primary friedel-Crafts reactions i.e., alkylation and acylation.
Example:
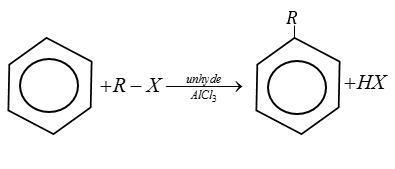
Complete step by step answer:
Let us write Friedel-Crafts reactions one by one with the given option.
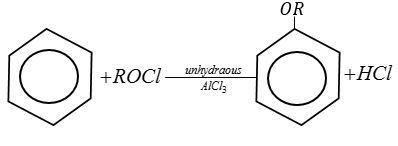
Diagram:
It takes place at a slower rate.
Friedel-Crafts reaction with acetophenone takes place at high pressure and not possible.
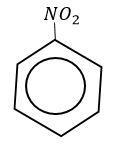
With nitrobenzene it is not possible because electrons are a withdrawing group present in the benzene ring.
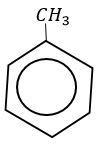
With toluene it takes place at a faster rate due to hyper conjugation.
Hyper conjugation is interaction of electrons in $\sigma $-bond (usually C-H or C-C) with an adjacent empty or partially filled P-orbital or $\pi $-orbital.
This increases the stability of molecules.
This is also known as no-bond.
This is a permanent effect.
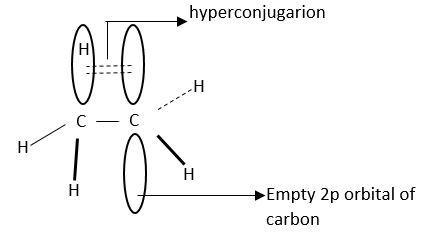
Hyperconjugation stabilizes carbocation as it helps in dispersal of positive charges.
As the number of $\alpha - H - $atoms increases. The relative stability on the basis of hyperconjugation increase
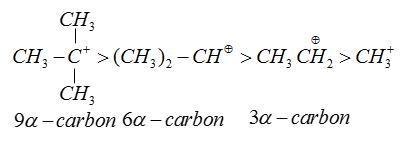
Therefore, form the above explanation the correct option toluene.
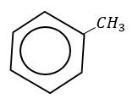
So, the correct answer is “Option D”.
Note:
Friedel-Crafts reactions always occur in the presence of anhydrous $AlC{l_3}. $Anhydrous $AlC{l_3}$ converts nucleophile and electrophile.
In Aromatic compounds only electrophilic substitution takes place.
There are two primary friedel-Crafts reactions i.e., alkylation and acylation.
Example:


Complete step by step answer:
Let us write Friedel-Crafts reactions one by one with the given option.
Diagram:
It takes place at a slower rate.
Friedel-Crafts reaction with acetophenone takes place at high pressure and not possible.

With nitrobenzene it is not possible because electrons are a withdrawing group present in the benzene ring.

With toluene it takes place at a faster rate due to hyper conjugation.
Hyper conjugation is interaction of electrons in $\sigma $-bond (usually C-H or C-C) with an adjacent empty or partially filled P-orbital or $\pi $-orbital.
This increases the stability of molecules.
This is also known as no-bond.
This is a permanent effect.

Hyperconjugation stabilizes carbocation as it helps in dispersal of positive charges.
As the number of $\alpha - H - $atoms increases. The relative stability on the basis of hyperconjugation increase
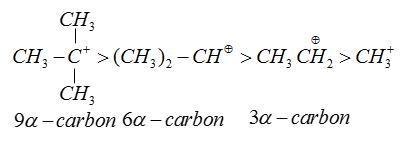
Therefore, form the above explanation the correct option toluene.

So, the correct answer is “Option D”.
Note:
Friedel-Crafts reactions always occur in the presence of anhydrous $AlC{l_3}. $Anhydrous $AlC{l_3}$ converts nucleophile and electrophile.
In Aromatic compounds only electrophilic substitution takes place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




