
What is the formula of a compound in which the element Y forms ccp lattice and atoms of X occupy ${{{}^{1}/{}_{3}}^{rd}}$ of tetrahedral voids?
Answer
595.2k+ views
Hint: To answer this question we should know that the number of elements forming a ccp lattice is 4 and the total number of tetrahedral voids is twice the number of the element forming the ccp lattice, that is 2 X 4 = 8.
Complete step by step solution:
Let’s look at the solution of the given problem:
A tetrahedral void is formed when four atoms are in contact with each-other. This happens when a sphere of a second layer is placed just above the void of the first layer. These are known as tetrahedral voids because the shape of the void formed resembles a tetrahedron.
Let’s look at the shape of the tetrahedral void.
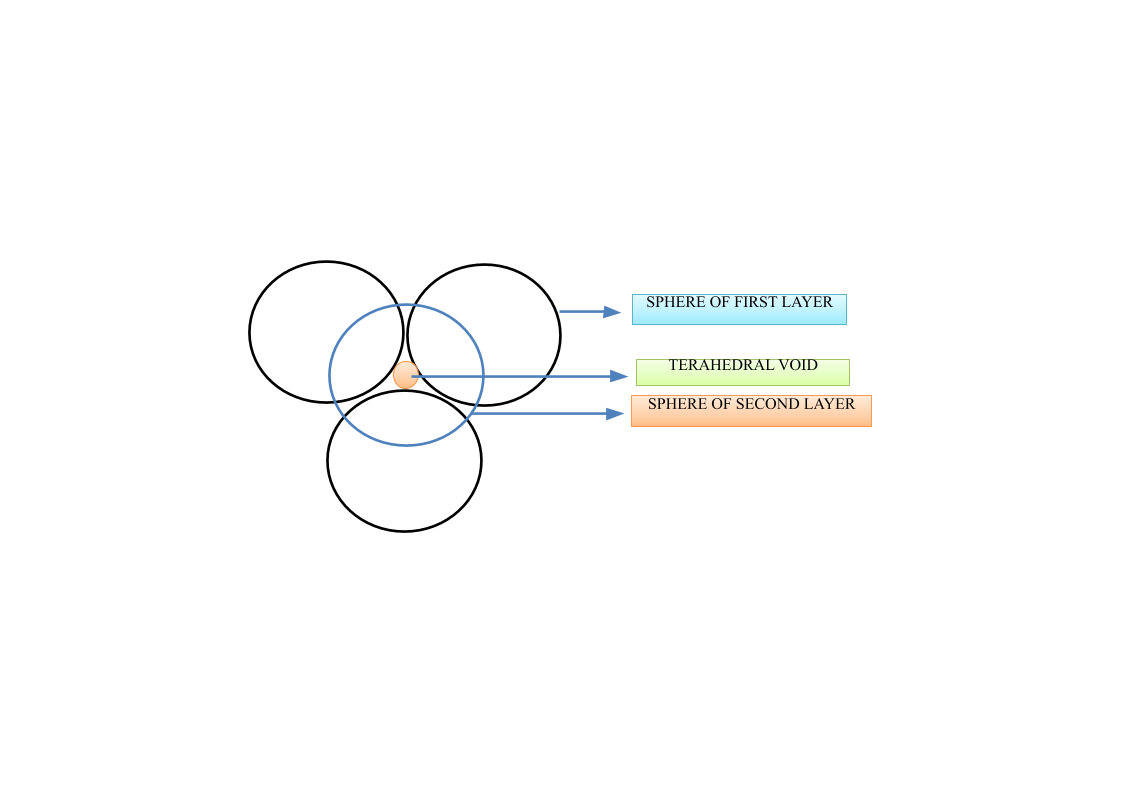
Since, Y forms the ccp lattice in the compound, so, the number of Y atoms per unit cell will bw:
Y= 4 atoms per unit cell
Now, we will calculate the number of tetrahedral voids in the lattice.
Number of tetrahedral voids is twice the number of Y atoms
Therefore, number of tetrahedral voids $=2\times 4=8$
Now, we will calculate the number of X atoms per unit cell
Now, it is given in the question that atoms of X occupy ${{{}^{1}/{}_{3}}^{rd}}$ of the tetrahedral voids
So, number of X atoms $=\,\dfrac{1}{3}\times 8\,=\,\dfrac{8}{3}$
X= $\dfrac{8}{3}$ atoms per unit cell
So, the formula of the compound will be ${{X}_{\dfrac{8}{3}}}{{Y}_{4}}$
Now, we will convert it into the simplest whole number ratio to get the formula of the compound.
\[\begin{align}
& \Rightarrow \dfrac{8}{3}\,=\,4 \\
& \Rightarrow \,\dfrac{2}{3}=1 \\
& \Rightarrow \dfrac{2}{3}\times 3\,=\,1\times 3 \\
& \Rightarrow 2=3 \\
& =2:3 \\
\end{align}\]
Therefore, the formula of the given compound will become ${{X}_{2}}{{Y}_{3}}$
Hence, the answer of the given question is ${{X}_{2}}{{Y}_{3}}$.
Note: This question is dependent on the type of lattice. So students must remember the number of atoms per unit cell for the different types of lattices like fcc, bcc, etc. For fcc it is 4 atoms per unit cell and for bcc it is 2 atoms per unit cell.
Complete step by step solution:
Let’s look at the solution of the given problem:
A tetrahedral void is formed when four atoms are in contact with each-other. This happens when a sphere of a second layer is placed just above the void of the first layer. These are known as tetrahedral voids because the shape of the void formed resembles a tetrahedron.
Let’s look at the shape of the tetrahedral void.

Since, Y forms the ccp lattice in the compound, so, the number of Y atoms per unit cell will bw:
Y= 4 atoms per unit cell
Now, we will calculate the number of tetrahedral voids in the lattice.
Number of tetrahedral voids is twice the number of Y atoms
Therefore, number of tetrahedral voids $=2\times 4=8$
Now, we will calculate the number of X atoms per unit cell
Now, it is given in the question that atoms of X occupy ${{{}^{1}/{}_{3}}^{rd}}$ of the tetrahedral voids
So, number of X atoms $=\,\dfrac{1}{3}\times 8\,=\,\dfrac{8}{3}$
X= $\dfrac{8}{3}$ atoms per unit cell
So, the formula of the compound will be ${{X}_{\dfrac{8}{3}}}{{Y}_{4}}$
Now, we will convert it into the simplest whole number ratio to get the formula of the compound.
\[\begin{align}
& \Rightarrow \dfrac{8}{3}\,=\,4 \\
& \Rightarrow \,\dfrac{2}{3}=1 \\
& \Rightarrow \dfrac{2}{3}\times 3\,=\,1\times 3 \\
& \Rightarrow 2=3 \\
& =2:3 \\
\end{align}\]
Therefore, the formula of the given compound will become ${{X}_{2}}{{Y}_{3}}$
Hence, the answer of the given question is ${{X}_{2}}{{Y}_{3}}$.
Note: This question is dependent on the type of lattice. So students must remember the number of atoms per unit cell for the different types of lattices like fcc, bcc, etc. For fcc it is 4 atoms per unit cell and for bcc it is 2 atoms per unit cell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




