
What is the formal charge on the \[Br\] and \[O\] atoms in the \[Br{{O}_{3}}^{-}\] ion?
Answer
513.6k+ views
Hint: In the structure of \[Br{{O}_{3}}^{-}\] , bromine is attached with two oxygen atoms through a double bond and is attached with one oxygen atom through a single bond. The formal charge can easily be found out by drawing Lewis structure of bromate ion.
Complete answer: The formal charge of \[Br\] (bromine) atoms in the \[Br{{O}_{3}}^{-}\] (bromate) ion is 0 and that of \[O\] (oxygen) is -1.
Understanding this with the help of Lewis structure of bromate ion will be much simpler.
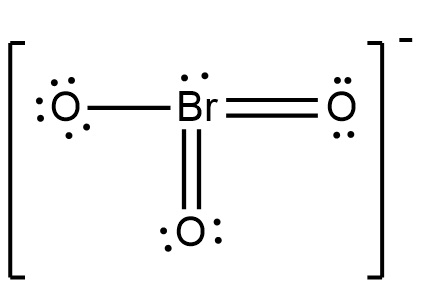
The above shown figure is the Lewis structure of bromate ions. Now, as shown in the figure above, one bromine atom is attached with three oxygen atoms. Two of those oxygen atoms are attached with bromine atoms through a double bond, and one oxygen atom is attached with a bromine atom through a single bond.
Now, as shown the oxygen attached to bromine with double bond has six electrons in its orbit, thus they are stable. But, the oxygen attached to bromine with a single bond has seven electrons in its orbit. Thus, the formal charge on the oxygen atom will be -1. And as bromine has 7 electrons around it, four from double bonded oxygen, one from single bonded oxygen and two lone pairs, it is also stable.
Note:
The Lewis structure of bromate ion originally is given as below.
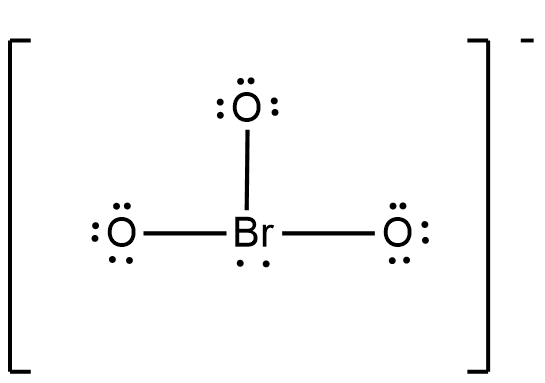
But, as this structure is not stable, two oxygen forms double bond with the bromine atom and makes the ion more stable. But still there is one oxygen atom with negative charge and thus the formal charge of oxygen is -1.
Complete answer: The formal charge of \[Br\] (bromine) atoms in the \[Br{{O}_{3}}^{-}\] (bromate) ion is 0 and that of \[O\] (oxygen) is -1.
Understanding this with the help of Lewis structure of bromate ion will be much simpler.

The above shown figure is the Lewis structure of bromate ions. Now, as shown in the figure above, one bromine atom is attached with three oxygen atoms. Two of those oxygen atoms are attached with bromine atoms through a double bond, and one oxygen atom is attached with a bromine atom through a single bond.
Now, as shown the oxygen attached to bromine with double bond has six electrons in its orbit, thus they are stable. But, the oxygen attached to bromine with a single bond has seven electrons in its orbit. Thus, the formal charge on the oxygen atom will be -1. And as bromine has 7 electrons around it, four from double bonded oxygen, one from single bonded oxygen and two lone pairs, it is also stable.
Note:
The Lewis structure of bromate ion originally is given as below.

But, as this structure is not stable, two oxygen forms double bond with the bromine atom and makes the ion more stable. But still there is one oxygen atom with negative charge and thus the formal charge of oxygen is -1.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




