
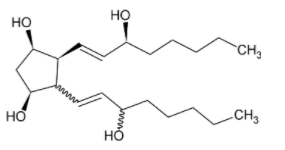
For the given compound $X$, the total number of optically active stereoisomers is _____.

Answer
548.4k+ views
Hint:
To answer this question, you must recall drawing stereoisomers of an organic compound. Stereoisomers are isomers that differ in spatial arrangement of atoms. Stereoisomers can be of two types, optically active stereoisomers and mesomeric isomers.
Complete step by step solution:
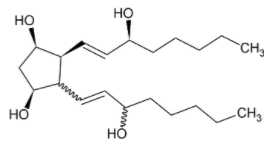
It is given in the question that the bond represented by a solid line indicates that the configuration of the specific carbon atom and the geometry of the double bond is fixed.
While the zigzag line indicates that the configuration of the specific carbon atom and the geometry of double bonds can vary.
So, to draw the stereoisomers of the given molecule, we can just vary the configuration of the zig-zag bonds.
The double bond can take two forms, namely cis and trans while keeping the orientation of bond 4 and bond 2 outside the plane.
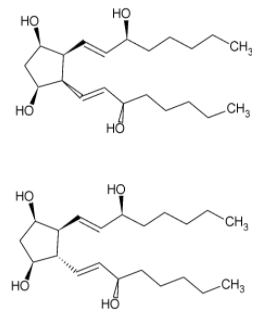
Now, changing the orientation of bond 4 inside the plane, we get two more cis and trans isomers.
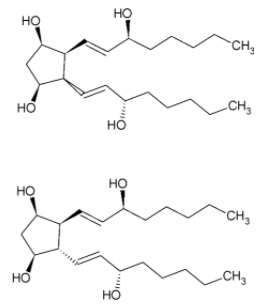
Similarly, we get 4 more isomers when the bond 2 is inside the plane
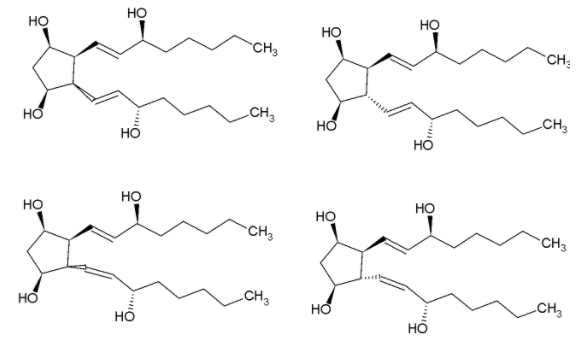
We get a total of 8 stereoisomers.
In stereoisomer 1, we can see a plane of symmetry in the molecule. Thus, it is a mesomer and not optically active. Rest of the stereoisomers are optically active due to the absence of any plane of symmetry, or axis of symmetry or any point of symmetry.
Therefore, the number of optically active stereoisomers of the given compound are 7.
Note:
The maximum number of stereoisomers possible in a compound can be given by a simple formula,
No. of stereoisomers$ = {2^n}$
Where, $n$ is the number of stereocenters present in the molecule.
In the above question, with the given conditions, the number of stereocenters are three.
As a result, the total number of stereoisomers possible can be given by ${2^3} = 8$
To answer this question, you must recall drawing stereoisomers of an organic compound. Stereoisomers are isomers that differ in spatial arrangement of atoms. Stereoisomers can be of two types, optically active stereoisomers and mesomeric isomers.
Complete step by step solution:

It is given in the question that the bond represented by a solid line indicates that the configuration of the specific carbon atom and the geometry of the double bond is fixed.
While the zigzag line indicates that the configuration of the specific carbon atom and the geometry of double bonds can vary.
So, to draw the stereoisomers of the given molecule, we can just vary the configuration of the zig-zag bonds.
The double bond can take two forms, namely cis and trans while keeping the orientation of bond 4 and bond 2 outside the plane.

Now, changing the orientation of bond 4 inside the plane, we get two more cis and trans isomers.

Similarly, we get 4 more isomers when the bond 2 is inside the plane

We get a total of 8 stereoisomers.
In stereoisomer 1, we can see a plane of symmetry in the molecule. Thus, it is a mesomer and not optically active. Rest of the stereoisomers are optically active due to the absence of any plane of symmetry, or axis of symmetry or any point of symmetry.
Therefore, the number of optically active stereoisomers of the given compound are 7.
Note:
The maximum number of stereoisomers possible in a compound can be given by a simple formula,
No. of stereoisomers$ = {2^n}$
Where, $n$ is the number of stereocenters present in the molecule.
In the above question, with the given conditions, the number of stereocenters are three.
As a result, the total number of stereoisomers possible can be given by ${2^3} = 8$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




