
For the following compounds, the correct statement (s) with respect to nucleophilic substitution reaction is are:
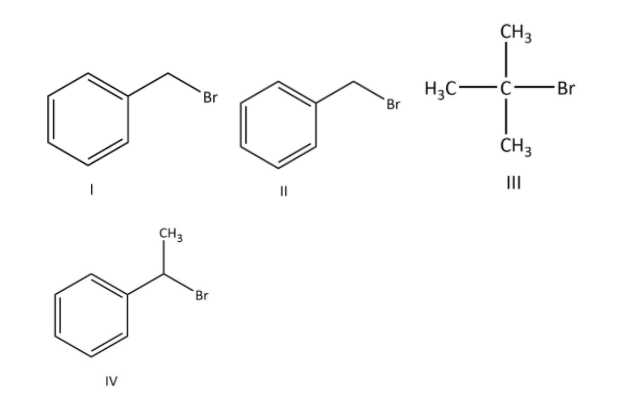
(A) \[{\rm{I}}\] and \[{\rm{III}}\] follow \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism
(B) Compound \[{\rm{IV}}\] undergoes inversion of configuration
(C) \[{\rm{I}}\] and \[{\rm{II}}\] follow \[{{\rm{S}}_{\rm{N}}}2\] mechanism
(D) The order of reactivity for \[{\rm{I}}\], \[{\rm{III}}\] and \[{\rm{IV}}\] is: \[{\rm{I}}\], \[{\rm{III}}\] and \[{\rm{IV}}\,{\rm{ > }}\,{\rm{I}}\,{\rm{ > }}\,{\rm{III}}\,\].

Answer
577.8k+ views
Hint: As we know that, the nucleophilic substitution reaction is of two types, one is unimolecular nucleophilic substitution reaction and bimolecular nucleophilic substitution reaction. Unimolecular nucleophilic substitution reaction depends upon only the reaction intermediate and bimolecular nucleophilic substitution reaction depends upon nucleophile as well as substrate.
Complete step by step answer
Unimolecular nucleophilic substitution reaction is represented as \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] and bimolecular nucleophilic substitution reaction represented as \[{{\rm{S}}_{\rm{N}}}2\].
\[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] reaction proceeds only when the carbocation intermediate is very stable. That’s why it is represented by one as it depends only on the carbocation intermediate. \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] reaction mechanism is completed by two steps, in which first step is to generate carbocation intermediate and then attack of nucleophile. Even weak nucleophiles can approach the carbocation intermediate. The stability of carbocation in groups follows the order is \[{\rm{benzyl}}\,\,{\rm{carbocation > }}\,{{\rm{3}}^{\rm{0}}}{\rm{ > }}{{\rm{2}}^{\rm{0}}}{\rm{ > }}{{\rm{1}}^{\rm{0}}}\].
\[{{\rm{S}}_{\rm{N}}}2\] reaction depends upon the concentration of the nucleophile and order of substrate. The kinetic order of the reaction is two and the mechanism is completed in only one step.
The order of the substrate in the alkyl group to attack the nucleophile is \[{1^0} > {2^0} > {3^0}\].
Now in the given options,
\[{\rm{I}}\] and \[{\rm{III}}\] follow \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism because \[{\rm{I}}\] and \[{\rm{III}}\] will form most stable carbocation as we have seen in our explanation.
In option (B), the \[{\rm{IV}}\] compound will undergo inversion in configuration by \[{{\rm{S}}_{\rm{N}}}2\] mechanism when the nucleophile approach to it.
In option (C), \[{\rm{I}}\] and \[{\rm{II}}\] will follow \[{{\rm{S}}_{\rm{N}}}2\] mechanism because both of the compounds contain primary carbon.
In option (D), the order of reactivity by \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism for \[{\rm{I}}\], \[{\rm{III}}\] and \[{\rm{IV}}\] is \[{\rm{IV}}\,{\rm{ > }}\,{\rm{I}}\,{\rm{ > }}\,{\rm{III}}\,\] as we have seen our explanation.
Therefore, the correct options are (A), (B), (C) and (D).
Note:
\[{{\rm{S}}_{\rm{N}}}2\] mechanism form products with inversion in configuration and \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism form products with retention in configuration.
Complete step by step answer
Unimolecular nucleophilic substitution reaction is represented as \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] and bimolecular nucleophilic substitution reaction represented as \[{{\rm{S}}_{\rm{N}}}2\].
\[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] reaction proceeds only when the carbocation intermediate is very stable. That’s why it is represented by one as it depends only on the carbocation intermediate. \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] reaction mechanism is completed by two steps, in which first step is to generate carbocation intermediate and then attack of nucleophile. Even weak nucleophiles can approach the carbocation intermediate. The stability of carbocation in groups follows the order is \[{\rm{benzyl}}\,\,{\rm{carbocation > }}\,{{\rm{3}}^{\rm{0}}}{\rm{ > }}{{\rm{2}}^{\rm{0}}}{\rm{ > }}{{\rm{1}}^{\rm{0}}}\].
\[{{\rm{S}}_{\rm{N}}}2\] reaction depends upon the concentration of the nucleophile and order of substrate. The kinetic order of the reaction is two and the mechanism is completed in only one step.
The order of the substrate in the alkyl group to attack the nucleophile is \[{1^0} > {2^0} > {3^0}\].
Now in the given options,
\[{\rm{I}}\] and \[{\rm{III}}\] follow \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism because \[{\rm{I}}\] and \[{\rm{III}}\] will form most stable carbocation as we have seen in our explanation.
In option (B), the \[{\rm{IV}}\] compound will undergo inversion in configuration by \[{{\rm{S}}_{\rm{N}}}2\] mechanism when the nucleophile approach to it.
In option (C), \[{\rm{I}}\] and \[{\rm{II}}\] will follow \[{{\rm{S}}_{\rm{N}}}2\] mechanism because both of the compounds contain primary carbon.
In option (D), the order of reactivity by \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism for \[{\rm{I}}\], \[{\rm{III}}\] and \[{\rm{IV}}\] is \[{\rm{IV}}\,{\rm{ > }}\,{\rm{I}}\,{\rm{ > }}\,{\rm{III}}\,\] as we have seen our explanation.
Therefore, the correct options are (A), (B), (C) and (D).
Note:
\[{{\rm{S}}_{\rm{N}}}2\] mechanism form products with inversion in configuration and \[{{\rm{S}}_{\rm{N}}}{\rm{1}}\] mechanism form products with retention in configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




