
For propadiene ${H_2}C = C = C{H_2}$ , correct statement(s) is/are:
A) Molecules are non planar.
B) Molecules are nonpolar.
C) Nodal plane of the pi bond formed by C1&C2 is perpendicular to that formed by C2&C3.
D) Nodal plane of pi bond formed by C1&C2 is Coplanar to that of formed by C2&C3
Answer
556.5k+ views
Hint: We must need to know that the allenes are in which one carbon molecule has twofold bonds with every one of its two neighboring carbon centres. Allenes are delegated cumulated dienes. The parent compound of this class is propadiene, which is itself likewise called allene. Mixes with an allene-type structure yet with in excess of three carbon particles are individuals from a bigger class of mixes called cumulenes with X=C=Y bonding.
Complete step by step answer:
We need to remember that the central atom of Allenes structures two sigma bonds and two pi bonds. The focal carbon is sp-hybridized, and the two terminal carbon molecules are \[s{p^2}\] hybridized. The bond point shaped by the three carbon atoms is \[180^\circ \], showing straight calculation for the focal carbon molecule. The two terminal carbon molecules are planar, and these planes are wound $90^\circ $ from one another. The structure can likewise be seen as a "broadened tetrahedral" with a comparative shape to methane, a similarity that is preceded into the stereochemical investigation of certain subsidiary atoms.
Let us see the structure of propadiene,
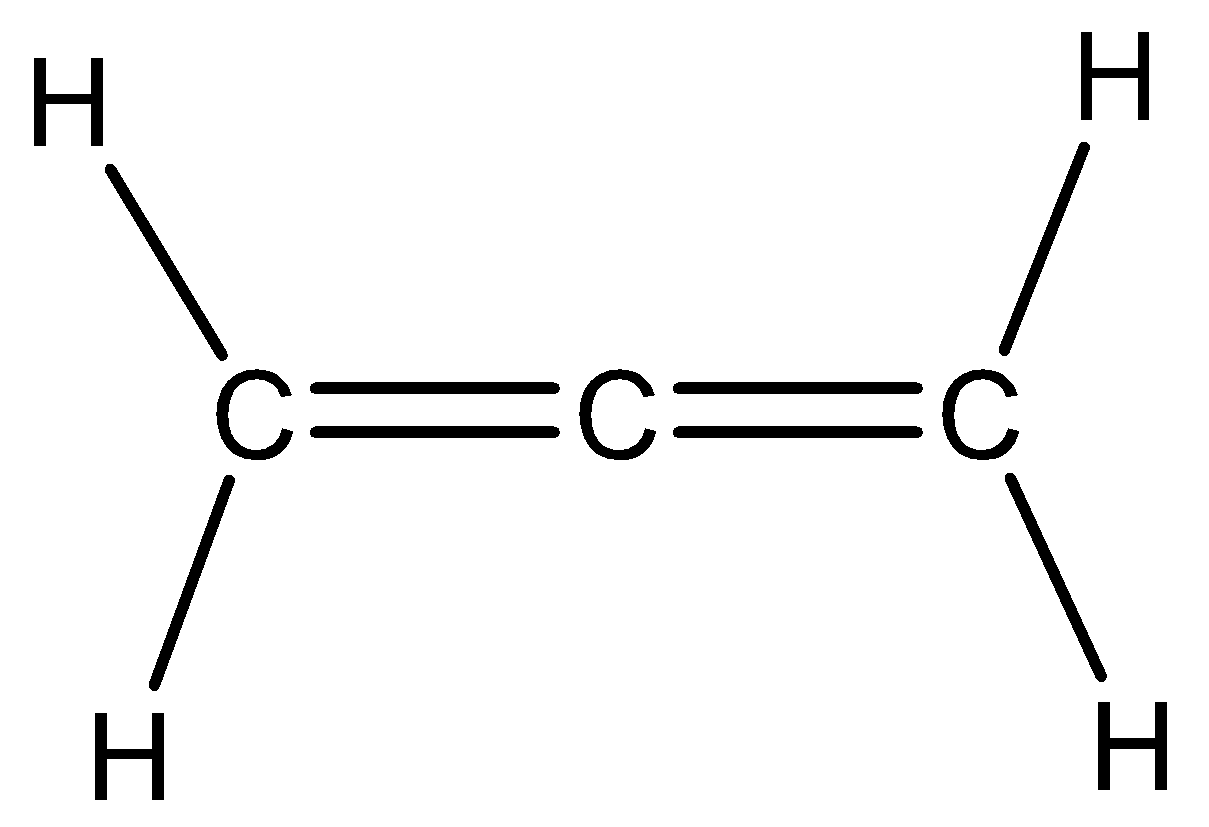
We need to know that the central atom in the above structure is sp hybridized since it has two pi bonds in addition to sigma bonds with two adjacent carbons. The remaining unhybridized p orbital is in perpendicular directions. Hence in propadiene the two methylene groups are in perpendicular planes. Hence option A and C are correct.
Note:
We must remember that hybridisation is the idea of blending nuclear orbitals into new mixture orbitals appropriate for the matching of electrons to frame compound bonds in valence bond hypothesis.
For instance, in a carbon iota which structures four single bonds the valence-shell s orbital joins with three valence-shell p orbitals to frame four comparable \[s{p^3}\] combinations which are masterminded in a tetrahedral plan around the carbon to cling to four distinct atoms. Hybrid orbitals are helpful in the clarification of sub-atomic math and nuclear holding properties and are evenly arranged in space. Generally half breed orbitals are shaped by blending nuclear orbitals of similar energies.
Complete step by step answer:
We need to remember that the central atom of Allenes structures two sigma bonds and two pi bonds. The focal carbon is sp-hybridized, and the two terminal carbon molecules are \[s{p^2}\] hybridized. The bond point shaped by the three carbon atoms is \[180^\circ \], showing straight calculation for the focal carbon molecule. The two terminal carbon molecules are planar, and these planes are wound $90^\circ $ from one another. The structure can likewise be seen as a "broadened tetrahedral" with a comparative shape to methane, a similarity that is preceded into the stereochemical investigation of certain subsidiary atoms.
Let us see the structure of propadiene,

We need to know that the central atom in the above structure is sp hybridized since it has two pi bonds in addition to sigma bonds with two adjacent carbons. The remaining unhybridized p orbital is in perpendicular directions. Hence in propadiene the two methylene groups are in perpendicular planes. Hence option A and C are correct.
Note:
We must remember that hybridisation is the idea of blending nuclear orbitals into new mixture orbitals appropriate for the matching of electrons to frame compound bonds in valence bond hypothesis.
For instance, in a carbon iota which structures four single bonds the valence-shell s orbital joins with three valence-shell p orbitals to frame four comparable \[s{p^3}\] combinations which are masterminded in a tetrahedral plan around the carbon to cling to four distinct atoms. Hybrid orbitals are helpful in the clarification of sub-atomic math and nuclear holding properties and are evenly arranged in space. Generally half breed orbitals are shaped by blending nuclear orbitals of similar energies.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




