
For nitromethane molecule, write structures (I) showing significant resonance
stabilization
(ii) Indicating tautomerism
Answer
566.4k+ views
Hint: Resonance structures are a set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges.
Complete Step by step answer: Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
A molecule or ion with such delocalized electrons is represented by several contributing structures
Tautomerization is moving around bonds while resonance is only moving around electrons. Technically two resonance structures are not two different structures.
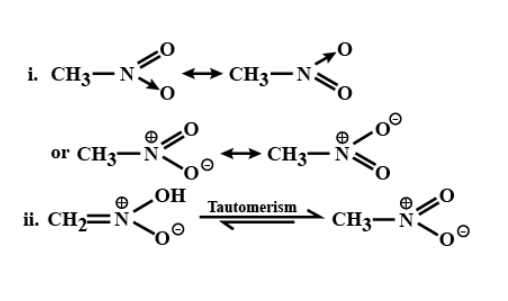
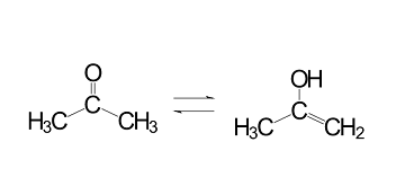
The most popular tautomerization is the enol and keto tautomers. With this type of tautomerization, we use a ketone or a molecule with a carbon double bonded to an oxygen. Although we typically show ketones in this manner, in reality, a solution of ketones will be switching back and forth in rapid equilibrium with another form, the enol. The enol moves a hydrogen from the alpha carbon onto the oxygen, moving the carbon-oxygen double bond to a carbon-carbon double bond.
Note: whenever the structure of a molecule cannot be represented by a single structure, but a combination of two or three structures in which there is distribution of charges on different molecules in different structures, the molecule is said to exhibit resonance. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula.
Complete Step by step answer: Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
A molecule or ion with such delocalized electrons is represented by several contributing structures
Tautomerization is moving around bonds while resonance is only moving around electrons. Technically two resonance structures are not two different structures.

The most popular tautomerization is the enol and keto tautomers. With this type of tautomerization, we use a ketone or a molecule with a carbon double bonded to an oxygen. Although we typically show ketones in this manner, in reality, a solution of ketones will be switching back and forth in rapid equilibrium with another form, the enol. The enol moves a hydrogen from the alpha carbon onto the oxygen, moving the carbon-oxygen double bond to a carbon-carbon double bond.

Note: whenever the structure of a molecule cannot be represented by a single structure, but a combination of two or three structures in which there is distribution of charges on different molecules in different structures, the molecule is said to exhibit resonance. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




