
For Cyclooctatetraene following is correct:
a. there are two types of C-C bond
B. structure is non planar and resonance is not observed in molecule
C. extensive resonance is found within the molecule and all bonds are of same type
D. the heat of hydrogenation is equal to that of 4π bonds hydrogenated.
Answer
559.5k+ views
Hint: Cyclooctatetraene is an anti-aromatic compound which is less stable than benzene.The structure of cyclooctatetraene is:
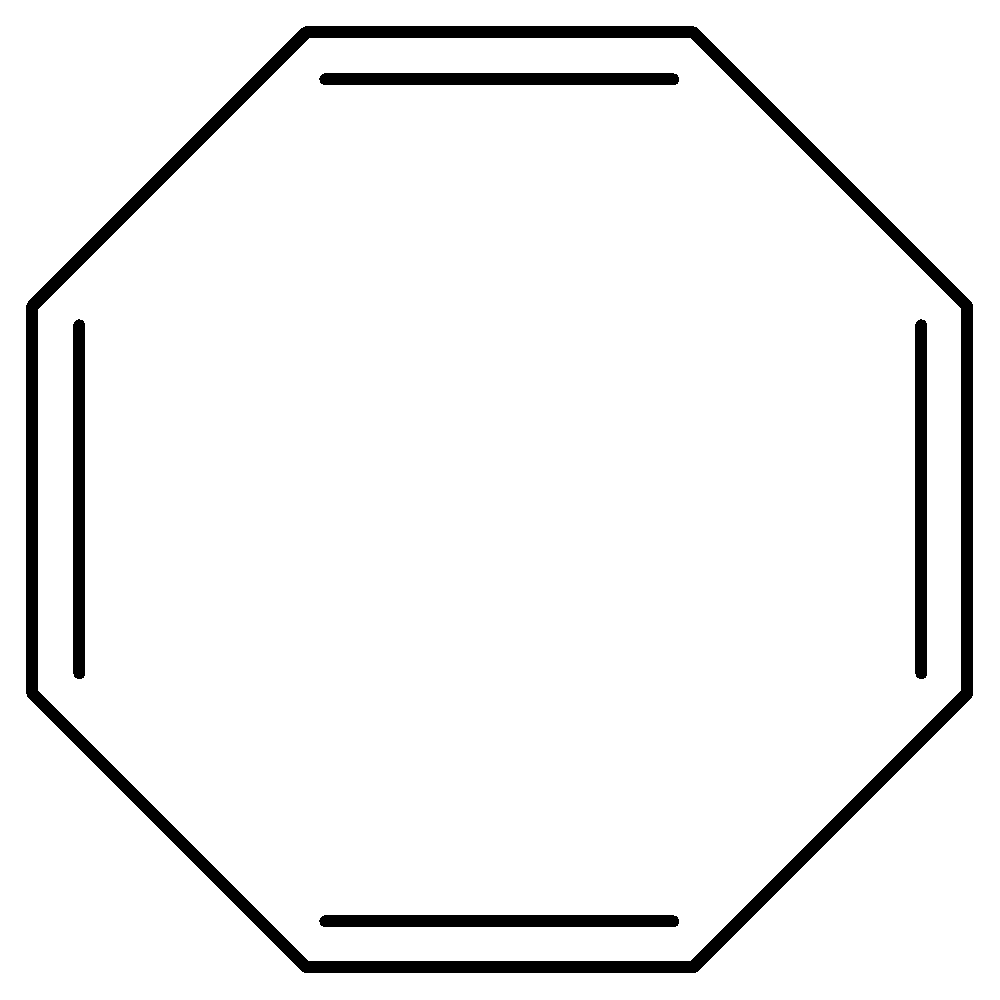
Complete step by step answer:
Cyclooctatetraene has \[{\text{8π }}\] electrons.
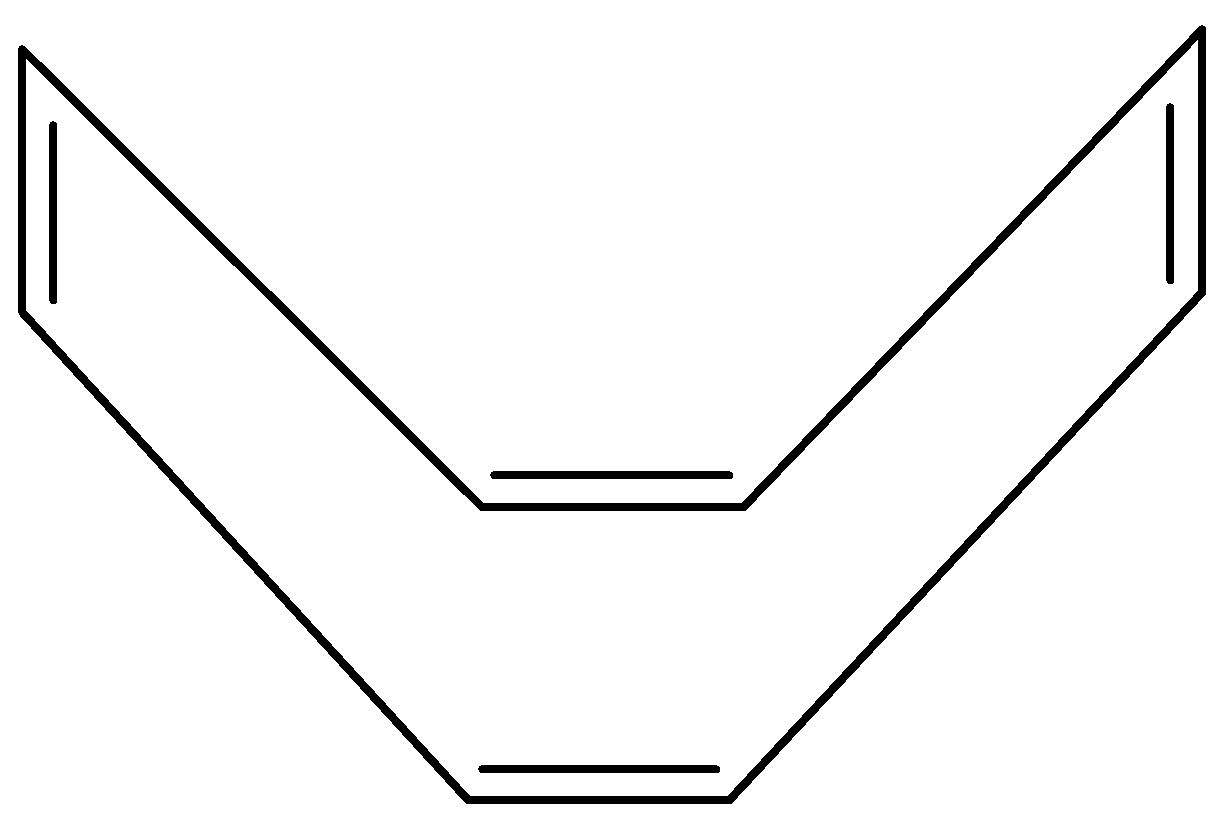
There are \[{\text{C - C}}\] single bonds and \[{\text{C = C}}\] double bonds in conjugation.
According to Hϋckel Rule only \[{\text{(4n + 2)}}\] electrons will allow a molecule to be aromatic and 8 is not the Hϋckel number but it fulfills all other criteria to be aromatic so it might be antiaromatic, but anti aromaticity is unstable, so cyclooctatetraene will bend and twist into tub shape to avoid anti aromaticity. By this shape, it becomes impossible for this molecule to overlap p-orbital which means that there will be no resonance in this molecule.
If the four double bonds of cyclooctatetraene are identical with that of cyclooctene its heat of hydrogenation is expected to be four times that of \[{\text{4π }}\] bonds hydrogenated.
Additional information: Hϋckel rule: if the number of π-electrons of monocyclic system is:
a. \[{\text{(4n + 2)}}\] then it is stable and aromatic.
b. \[{\text{4n}}\] it is unstable and anti aromatic.
Note:
Aromaticity is decided by four structural criteria:
1. Cyclic: each p-orbital overlap with the adjacent p-orbital.
2. Planar: p-orbitals must be aligned.
3. Completely Conjugated: p-orbital on every atom.
4. Satisfy Hϋckel rule: contain \[{\text{(4n + 2)π }}\] electrons.

Complete step by step answer:
Cyclooctatetraene has \[{\text{8π }}\] electrons.

There are \[{\text{C - C}}\] single bonds and \[{\text{C = C}}\] double bonds in conjugation.
According to Hϋckel Rule only \[{\text{(4n + 2)}}\] electrons will allow a molecule to be aromatic and 8 is not the Hϋckel number but it fulfills all other criteria to be aromatic so it might be antiaromatic, but anti aromaticity is unstable, so cyclooctatetraene will bend and twist into tub shape to avoid anti aromaticity. By this shape, it becomes impossible for this molecule to overlap p-orbital which means that there will be no resonance in this molecule.
If the four double bonds of cyclooctatetraene are identical with that of cyclooctene its heat of hydrogenation is expected to be four times that of \[{\text{4π }}\] bonds hydrogenated.
Additional information: Hϋckel rule: if the number of π-electrons of monocyclic system is:
a. \[{\text{(4n + 2)}}\] then it is stable and aromatic.
b. \[{\text{4n}}\] it is unstable and anti aromatic.
Note:
Aromaticity is decided by four structural criteria:
1. Cyclic: each p-orbital overlap with the adjacent p-orbital.
2. Planar: p-orbitals must be aligned.
3. Completely Conjugated: p-orbital on every atom.
4. Satisfy Hϋckel rule: contain \[{\text{(4n + 2)π }}\] electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




