
For a linear plot of \[\log (x/m)\] versus \[\log p\] in a Freundlich adsorption isotherm, which of the following statements is correct ? (\[k\]and \[n\] are constants)
A. \[\dfrac{1}{n}\] appears as the intercept.
B. Only \[\dfrac{1}{n}\] appears as the slope.
C. log\[\dfrac{1}{n}\] appears as the intercept.
D. Both \[k\] and \[\dfrac{1}{n}\] appear in the slope term
Answer
576k+ views
Hint: An adsorption isotherm is a curve relating the equilibrium concentration of a solute on the surface of an adsorbent. The adsorption isotherm is also an equation relating the amount of solute adsorbed onto the solid.
Complete step by step answer:
Freundlich Adsorption Isotherm gives the variation in the quantity of gas adsorbed by a unit mass of solid adsorbent with the change in pressure of the system for a given temperature. The expression for the Freundlich isotherm can be represented by the following equation;
\[\Rightarrow \dfrac{x}{m}=k{{P}^{\dfrac{1}{n}}}\]
Where \[x\] is the mass of the gas adsorbed, \[m\]is the mass of the adsorbent, \[P\] is the pressure and \[n\] is a constant which depends upon the nature of adsorbent and the gas at a given temperature. Taking the logarithm on both the sides of the equation, we get;
\[\Rightarrow \log \dfrac{x}{m}=\log k+\dfrac{1}{n}\log {{P}^{{}}}\]
On comparing this equation with the equation of straight line(\[y=mx+c\]). The plot of this equation is a straight line as represented by the following curve.
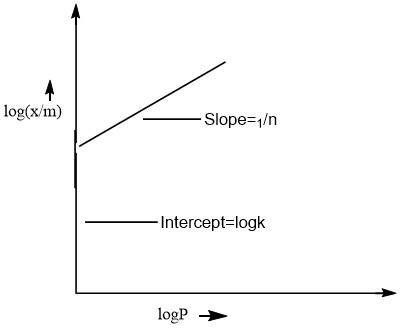
From the graph, it is very clear that only \[1/n\] appears as the slope.
So, the correct answer is Option B.
Note: The Freundlich adsorption isotherm is followed by another two isotherms, Langmuir adsorption isotherms and BET theory. The Langmuir adsorption isotherms predict linear adsorption at low adsorption densities and a maximum surface coverage at higher solute metal concentrations.
Complete step by step answer:
Freundlich Adsorption Isotherm gives the variation in the quantity of gas adsorbed by a unit mass of solid adsorbent with the change in pressure of the system for a given temperature. The expression for the Freundlich isotherm can be represented by the following equation;
\[\Rightarrow \dfrac{x}{m}=k{{P}^{\dfrac{1}{n}}}\]
Where \[x\] is the mass of the gas adsorbed, \[m\]is the mass of the adsorbent, \[P\] is the pressure and \[n\] is a constant which depends upon the nature of adsorbent and the gas at a given temperature. Taking the logarithm on both the sides of the equation, we get;
\[\Rightarrow \log \dfrac{x}{m}=\log k+\dfrac{1}{n}\log {{P}^{{}}}\]
On comparing this equation with the equation of straight line(\[y=mx+c\]). The plot of this equation is a straight line as represented by the following curve.

From the graph, it is very clear that only \[1/n\] appears as the slope.
So, the correct answer is Option B.
Note: The Freundlich adsorption isotherm is followed by another two isotherms, Langmuir adsorption isotherms and BET theory. The Langmuir adsorption isotherms predict linear adsorption at low adsorption densities and a maximum surface coverage at higher solute metal concentrations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




