
For a first-order reaction:
$A(g)\rightleftharpoons 3B(g)$
The concentration versus time graph is given below:
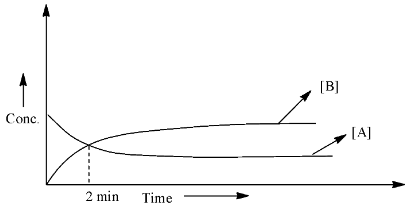
What is the half-life in minutes (answer to the nearest integer)?

Answer
533.4k+ views
Hint: Half-life is the time when the concentration of the reactant becomes half of the concentration taken at the starting of the reaction. From the graph, we can calculate the half-life when the concentration of the reactant is equal to the concentration of the product.
Complete answer: The given reaction is:
$A(g)\rightleftharpoons 3B(g)$
And the question says that this reaction is first order, which means that the rate of the reaction depends only on the concentration of one reactant.
Half-life is the time when the concentration of the reactant becomes half of the concentration taken at the starting of the reaction. From the graph, we can calculate the half-life when the concentration of the reactant is equal to the concentration of the product.
So, the given graph is:
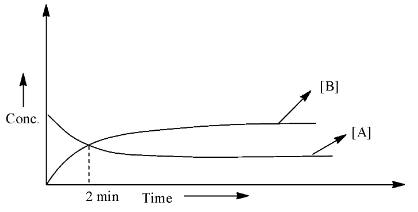
As we can see that the starting concentration of A is the highest and the concentration of B is zero. After 2 minutes, both the lines meet, which means that the concentration of the reactant and the product is equal. Therefore, half of the concentration of the A has been converted into a product, hence, this should be the half-life for the reaction.
Therefore, the half-life of the reaction is 2 min and this answer is already in the form of an integer.
Note: If the graph is not given for a reaction, but the rate constant, i.e., k is given for any first-order reaction, then we can calculate the half-life of the reaction by using the formula:
${{t}_{1/2}}=\dfrac{0693}{k}$
Complete answer: The given reaction is:
$A(g)\rightleftharpoons 3B(g)$
And the question says that this reaction is first order, which means that the rate of the reaction depends only on the concentration of one reactant.
Half-life is the time when the concentration of the reactant becomes half of the concentration taken at the starting of the reaction. From the graph, we can calculate the half-life when the concentration of the reactant is equal to the concentration of the product.
So, the given graph is:

As we can see that the starting concentration of A is the highest and the concentration of B is zero. After 2 minutes, both the lines meet, which means that the concentration of the reactant and the product is equal. Therefore, half of the concentration of the A has been converted into a product, hence, this should be the half-life for the reaction.
Therefore, the half-life of the reaction is 2 min and this answer is already in the form of an integer.
Note: If the graph is not given for a reaction, but the rate constant, i.e., k is given for any first-order reaction, then we can calculate the half-life of the reaction by using the formula:
${{t}_{1/2}}=\dfrac{0693}{k}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




