
Fluorobenzene (\[{C_6}{H_5}F\]) can be synthesised in the lab:
A.By heating phenol with \[HF\]and \[KF\]
B.From aniline by diazotization followed by heating the diazonium salt with \[HB{F_4}\]
C.By direct fluorination of benzene with \[{F_2}\]gas
D.By reacting bromo benzene with \[NaF\]solution.
Answer
589.5k+ views
Hint: First we have to discuss the structure of Fluorobenzene. Therefore, the name suggest it’s a molecule with Fluorine attached to a benzene having a chemical formula of \[{C_6}{H_5}F\].The process of synthesising of fluorobenzene contains the process of diazotization.
Complete step by step answer:
The structure of Fluorobenzene as follow:
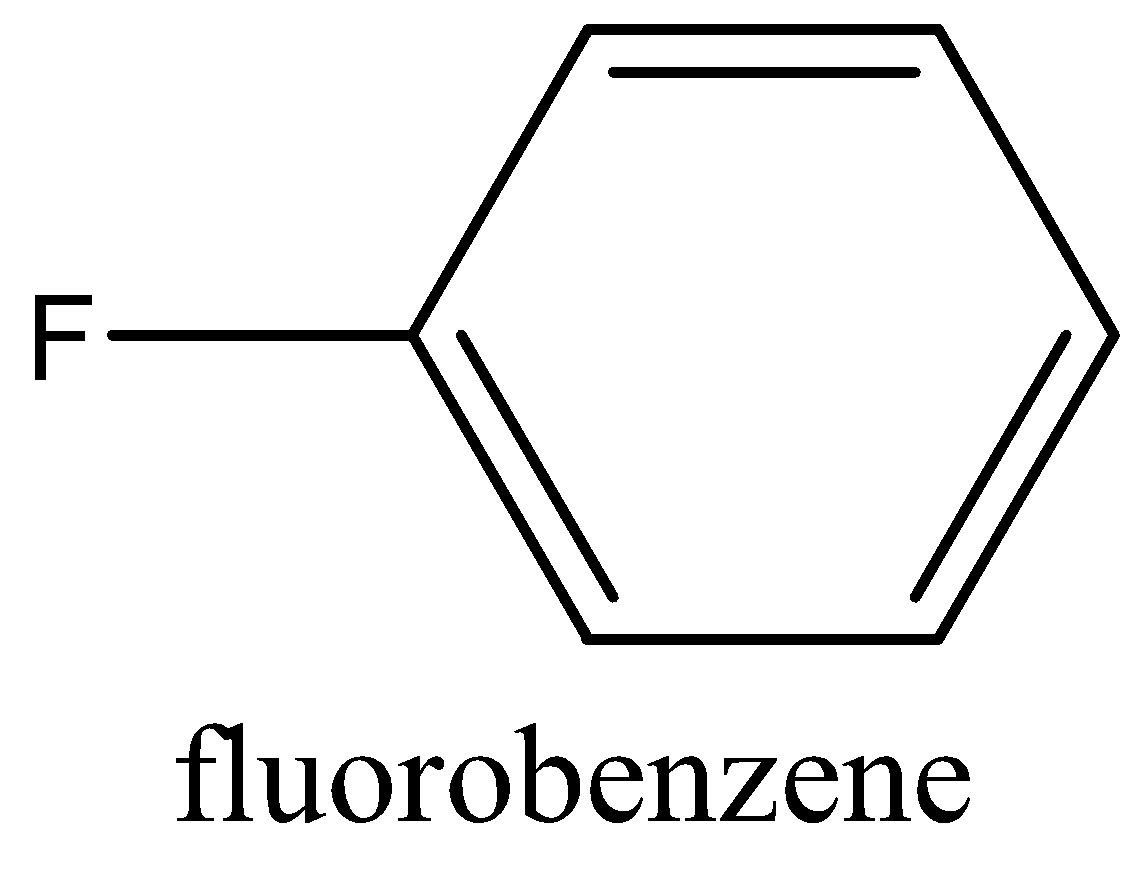
Now coming to the question we are asked how fluorobenzene can be synthesized. To synthesis fluorobenzene we first need to have aniline. The aniline is then converted into a diazonium salt with the process known as diazotization. In diazotization the aromatic amine like aniline is converted to a diazonium salt. The diazonium salt is having a chemical formula of \[R - {N_2}Cl\], R is a benzene ring. After getting diazonium salt we heat it with \[HB{F_4}\]to get the required product that is fluorobenzene. The reaction is given below:
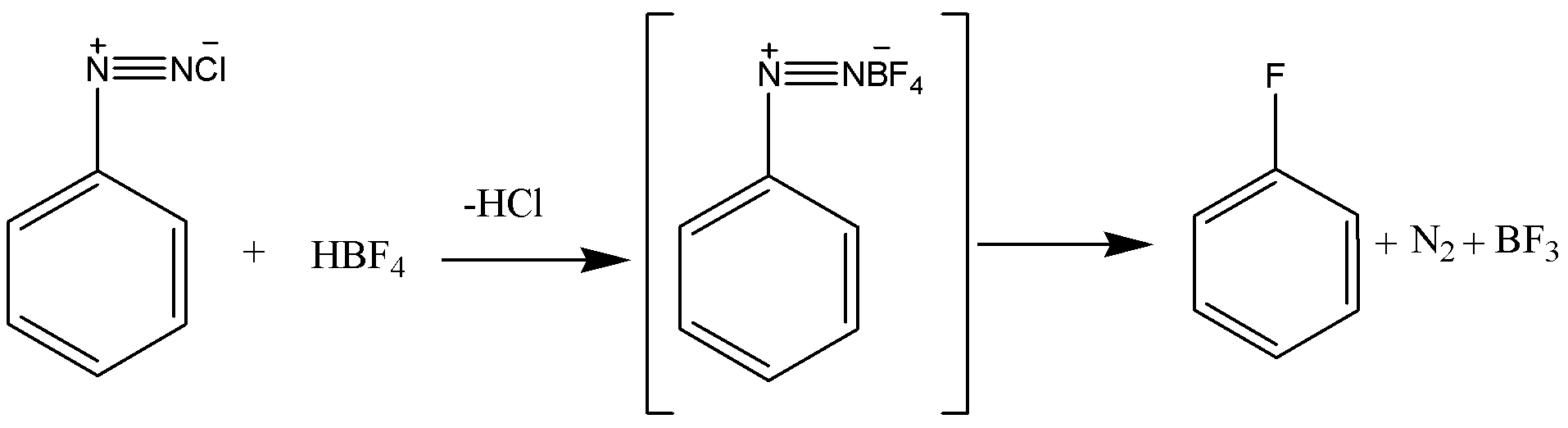
Hence the answer to this question is option B. From aniline by diazotization followed by heating the diazonium salt with \[HB{F_4}\]
Note:
We can use Fluorobenzene as a solvent for highly reactive species. It is to be noted that this molecule's melting point is even below that of benzene. The melting point of Fluorobenzene is $-44^oC$. Also the difference in boiling point between the two elements is only $4^oC$. This molecule is more polar than that .
Complete step by step answer:
The structure of Fluorobenzene as follow:

Now coming to the question we are asked how fluorobenzene can be synthesized. To synthesis fluorobenzene we first need to have aniline. The aniline is then converted into a diazonium salt with the process known as diazotization. In diazotization the aromatic amine like aniline is converted to a diazonium salt. The diazonium salt is having a chemical formula of \[R - {N_2}Cl\], R is a benzene ring. After getting diazonium salt we heat it with \[HB{F_4}\]to get the required product that is fluorobenzene. The reaction is given below:

Hence the answer to this question is option B. From aniline by diazotization followed by heating the diazonium salt with \[HB{F_4}\]
Note:
We can use Fluorobenzene as a solvent for highly reactive species. It is to be noted that this molecule's melting point is even below that of benzene. The melting point of Fluorobenzene is $-44^oC$. Also the difference in boiling point between the two elements is only $4^oC$. This molecule is more polar than that .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




