
Find ‘X’ in the given reaction.
${C_2}{H_5} - O - {C_2}{H_5} + CO\xrightarrow[{{{150}^o}C}]{{B{F_3}}}X$
A. $C{H_3}COOH$
B. $C{H_3}COO{C_2}{H_5}$
C. $C{H_3}C{H_2}COO{C_2}{H_5}$
D. ${C_3}{H_7}COO{C_2}{H_5}$
Answer
513.3k+ views
Hint: Insertion reaction: In organic chemistry, when a chemical compound, molecule interposes itself into an existing bond of another molecule, then the reaction is known as insertion reaction. The general equation for insertion reaction is represented as follows:
$A + B - C \to B - A - C$
Complete answer: For the given reaction conditions, $B{F_3}$ catalyses the reaction and on heating the given ether in the presence of carbon monoxide, the insertion reaction takes place and the atoms of carbon monoxide are inserted between the $C - O$ bond in ether and we get a yield of ester as a product. The reaction mechanism is given as follows:
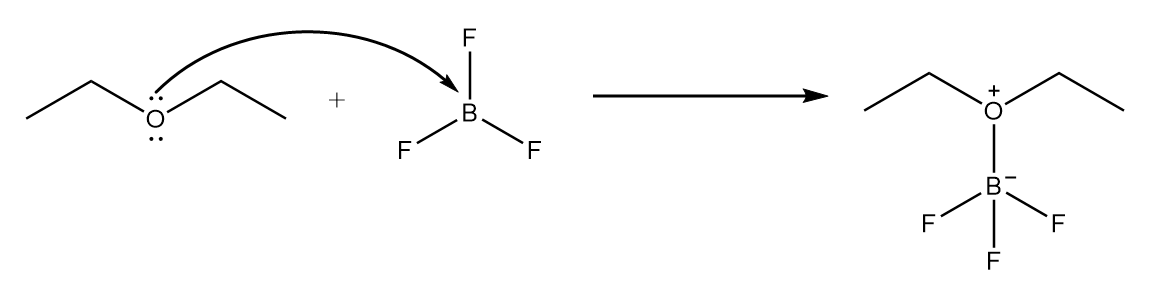
Step-1: Lone pair of electrons on oxygen atom present in ether attack $B{F_3}$ molecule and an intermediate product is formed. The reaction is shown as follows:
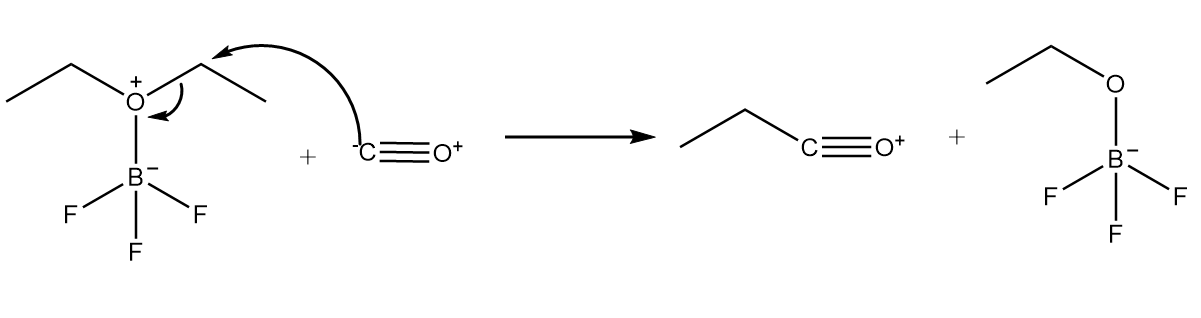
Step-2: Carbon atom of carbon monoxide attacks the carbon atom placed at adjacent position to oxygen atom and cleavage of $C - O$ bond takes place. The reaction proceeds as follows:
Step-3: As the positive charge on oxygen atoms makes the compound very unstable, so the cleavage of triple bond will take place and respective carbocation will be formed. The reaction proceeds as follows:
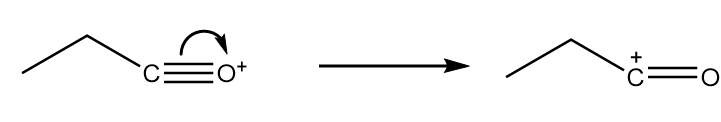
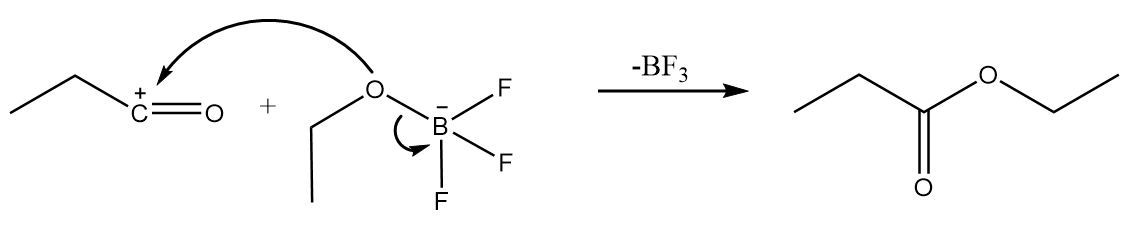
Step-4: The attack of $ - O{C_2}{H_5}$ group from the anion formed in step-2 takes place on carbocation along with the removal of $B{F_3}$ molecule. The reaction proceeds as follows:
Hence, in the given reaction sequence, the structure of the product formed i.e., X is $C{H_3}C{H_2}COO{C_2}{H_5}$.
So, option (C) is the correct answer.
Note:
It is important to note that $B{F_3}$ in the reaction acts as a neutral electrophile because it has six electrons in its valence shell and is considered as the electron deficient species due to its incomplete octet. Also, as the $B{F_3}$ molecule is produced as the by-product in the final step of the reaction therefore it is considered as a catalyst for the reaction.
$A + B - C \to B - A - C$
Complete answer: For the given reaction conditions, $B{F_3}$ catalyses the reaction and on heating the given ether in the presence of carbon monoxide, the insertion reaction takes place and the atoms of carbon monoxide are inserted between the $C - O$ bond in ether and we get a yield of ester as a product. The reaction mechanism is given as follows:
Step-1: Lone pair of electrons on oxygen atom present in ether attack $B{F_3}$ molecule and an intermediate product is formed. The reaction is shown as follows:

Step-2: Carbon atom of carbon monoxide attacks the carbon atom placed at adjacent position to oxygen atom and cleavage of $C - O$ bond takes place. The reaction proceeds as follows:

Step-3: As the positive charge on oxygen atoms makes the compound very unstable, so the cleavage of triple bond will take place and respective carbocation will be formed. The reaction proceeds as follows:
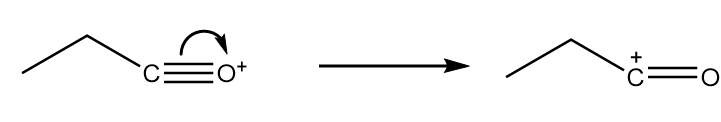
Step-4: The attack of $ - O{C_2}{H_5}$ group from the anion formed in step-2 takes place on carbocation along with the removal of $B{F_3}$ molecule. The reaction proceeds as follows:

Hence, in the given reaction sequence, the structure of the product formed i.e., X is $C{H_3}C{H_2}COO{C_2}{H_5}$.
So, option (C) is the correct answer.
Note:
It is important to note that $B{F_3}$ in the reaction acts as a neutral electrophile because it has six electrons in its valence shell and is considered as the electron deficient species due to its incomplete octet. Also, as the $B{F_3}$ molecule is produced as the by-product in the final step of the reaction therefore it is considered as a catalyst for the reaction.
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE

How many states of matter are there in total class 12 chemistry CBSE




