
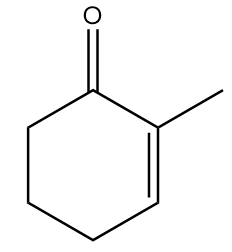
Find X in the following reaction.
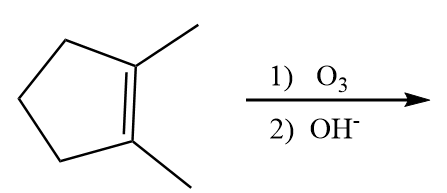
A.
B.
C.
D.





Answer
513.3k+ views
Hint: In organic reactions, when a compound having unsaturated bond i.e., double or triple bond, reacts with ozone then the cleavage of unsaturated bond takes place and carbonyl groups are introduced to the compound. So, after ozonolysis of unsaturated compounds, respective carbonyl compounds are formed as a product.
Complete answer: In the given reaction sequence, 1,2-dimethylcyclopent-1-ene undergoes ozonolysis followed by the aldol condensation in the presence of base. The mechanism for the reaction sequence is as follows:
Ozonolysis of 1,2-dimethylcyclopent-1-ene:
Step-1: Cleavage of double bond:
When ozone reacts with 1,2-dimethylcyclopent-1-ene, the double bond breaks and formation of an ozone layer takes place. The reaction proceeds as follows:
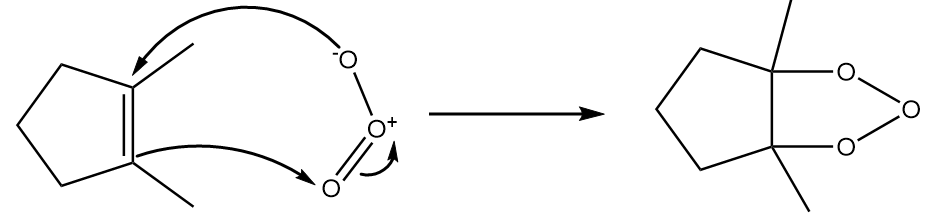
Step-2: Collapse of ring.
As the ozonide formed is unstable, so the ring collapses to form respective carbonyl compounds. The reaction takes place as follows:
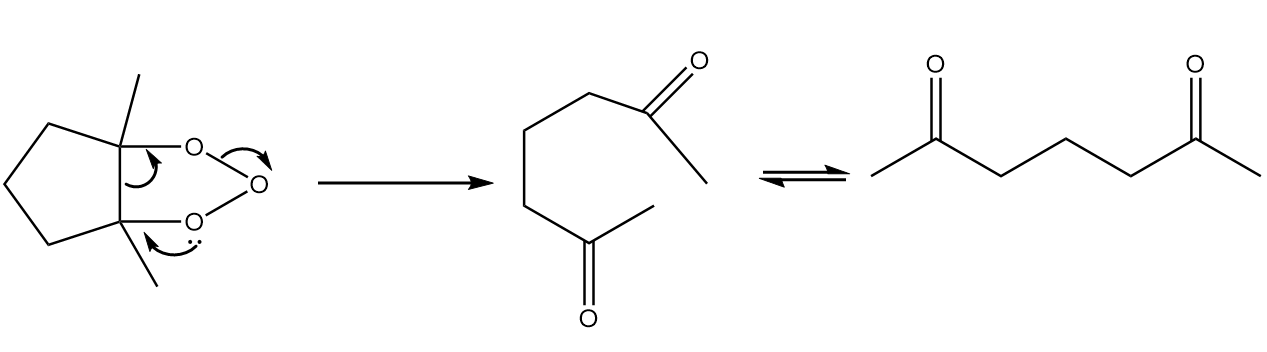
Now, in the presence of base i.e., $O{H^ - }$, intramolecular aldol condensation will take place as follows:
Step-1: The base extracts the alpha hydrogen of the molecule and carbanion is formed as an intermediate. The reaction takes place as follows:
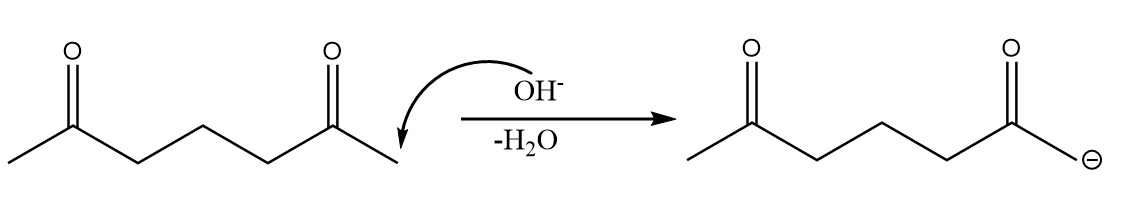
Step-2: The carbanion formed will attack the electrophilic carbonyl centre and formation of ring will take place. The reaction proceeds as follows:
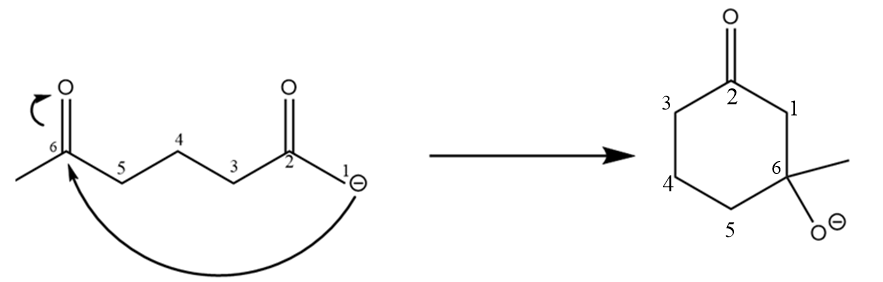
Step-3: Hydrogen ions will attack and respective alcohol will be formed. The reaction takes place as follows:
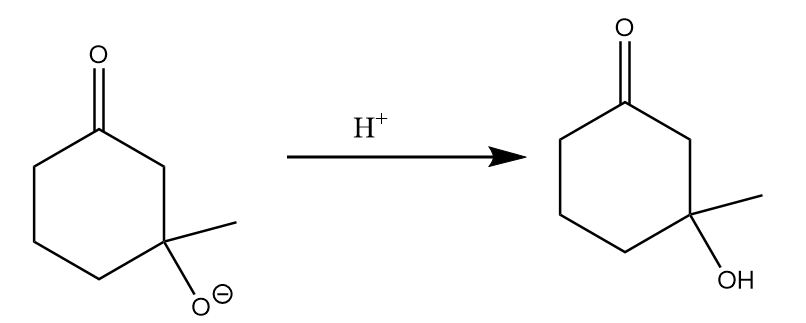
Step-4: Under warm conditions, removal of the water molecule takes place and the final product is formed. The reaction proceeds as follows:
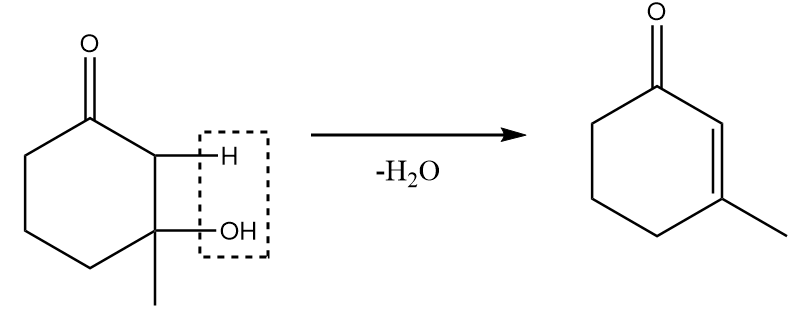
Hence, X in the given reaction sequence is as follows:
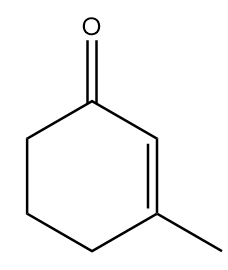
So, option (D) is the correct answer.
Note:
It is important to note that for a compound to undergo aldol condensation, it must have an alpha hydrogen. If there is no alpha hydrogen present in the compound then instead of aldol condensation, then under the same conditions, the Cannizzaro reaction will take place.
Complete answer: In the given reaction sequence, 1,2-dimethylcyclopent-1-ene undergoes ozonolysis followed by the aldol condensation in the presence of base. The mechanism for the reaction sequence is as follows:
Ozonolysis of 1,2-dimethylcyclopent-1-ene:
Step-1: Cleavage of double bond:
When ozone reacts with 1,2-dimethylcyclopent-1-ene, the double bond breaks and formation of an ozone layer takes place. The reaction proceeds as follows:
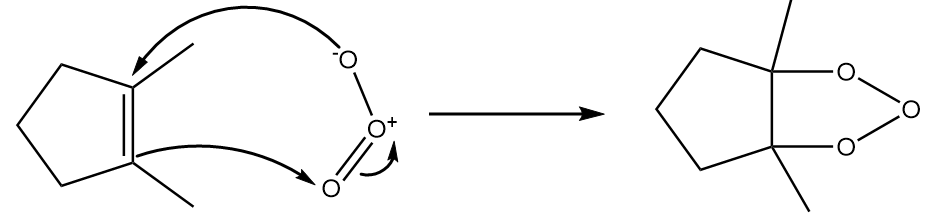
Step-2: Collapse of ring.
As the ozonide formed is unstable, so the ring collapses to form respective carbonyl compounds. The reaction takes place as follows:

Now, in the presence of base i.e., $O{H^ - }$, intramolecular aldol condensation will take place as follows:
Step-1: The base extracts the alpha hydrogen of the molecule and carbanion is formed as an intermediate. The reaction takes place as follows:

Step-2: The carbanion formed will attack the electrophilic carbonyl centre and formation of ring will take place. The reaction proceeds as follows:

Step-3: Hydrogen ions will attack and respective alcohol will be formed. The reaction takes place as follows:

Step-4: Under warm conditions, removal of the water molecule takes place and the final product is formed. The reaction proceeds as follows:

Hence, X in the given reaction sequence is as follows:

So, option (D) is the correct answer.
Note:
It is important to note that for a compound to undergo aldol condensation, it must have an alpha hydrogen. If there is no alpha hydrogen present in the compound then instead of aldol condensation, then under the same conditions, the Cannizzaro reaction will take place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




